Paternal age effects on sperm FOXK1 and KCNA7 methylation and transmission into the next generation
- PMID: 28171595
- PMCID: PMC5418740
- DOI: 10.1093/hmg/ddw328
Paternal age effects on sperm FOXK1 and KCNA7 methylation and transmission into the next generation
Abstract
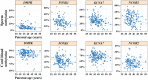
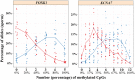
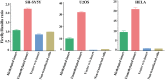
Children of older fathers carry an increased risk for developing autism and other disorders. To elucidate the underlying mechanisms, we investigated the correlation of sperm DNA methylation with paternal age and its impact on the epigenome of the offspring. Methylation levels of nine candidate genes and LINE-1 repeats were quantified by bisulfite pyrosequencing in sperm DNA of 162 donors and 191 cord blood samples of resulting children (conceived by IVF/ICSI with the same sperm samples). Four genes showed a significant negative correlation between sperm methylation and paternal age. For FOXK1 and KCNA7, the age effect on the sperm epigenome was replicated in an independent cohort of 188 sperm samples. For FOXK1, paternal age also significantly correlated with foetal cord blood (FCB) methylation. Deep bisulfite sequencing and allele-specific pyrosequencing allowed us to distinguish between maternal and paternal alleles in FCB samples with an informative SNP. FCB methylation of the paternal FOXK1 allele was negatively correlated with paternal age, whereas maternal allele was unaffected by maternal age. Since FOXK1 duplication has been associated with autism, we studied blood FOXK1 methylation in 74 children with autism and 41 age-matched controls. The FOXK1 promoter showed a trend for accelerated demethylation in the autism group. Dual luciferase reporter assay revealed that FOXK1 methylation influences gene expression. Collectively, our study demonstrates that age-related DNA methylation changes in sperm can be transmitted to the next generation and may contribute to the increased disease risk in offspring of older fathers.
Figures

References
-
- Nugent D., Balen A.H. (2001) The effects of female age on fecundity and pregnancy outcome. Hum. Fertil. (Camb.), 4, 43–48. - PubMed
-
- Frans E.M., Sandin S., Reichenberg A., Lichtenstein P., Langstrom N., Hultman C.M. (2008) Advancing paternal age and bipolar disorder. Arch. Gen. Psychiatry, 65, 1034–1040. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

