Macrophage Functions in Tissue Patterning and Disease: New Insights from the Fly
- PMID: 28171746
- PMCID: PMC5300050
- DOI: 10.1016/j.devcel.2017.01.001
Macrophage Functions in Tissue Patterning and Disease: New Insights from the Fly
Abstract
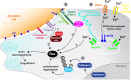
Macrophages are multifunctional innate immune cells that seed all tissues within the body and play disparate roles throughout development and in adult tissues, both in health and disease. Their complex developmental origins and many of their functions are being deciphered in mammalian tissues, but opportunities for live imaging and the genetic tractability of Drosophila are offering complementary insights into how these fascinating cells integrate a multitude of guidance cues to fulfill their many tasks and migrate to distant sites to either direct developmental patterning or raise an inflammatory response.
Keywords: Drosophila; apoptosis; development; disease; immunity; inflammation; macrophage; migration; wound.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Agaisse H., Petersen U.M., Boutros M., Mathey-Prevot B., Perrimon N. Signaling role of hemocytes in Drosophila JAK/STAT-dependent response to septic injury. Dev. Cell. 2003;5:441–450. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

