Protocol for the ENCODE trial: evaluating a novel online depression intervention for persons with epilepsy
- PMID: 28173780
- PMCID: PMC5297128
- DOI: 10.1186/s12888-017-1229-y
Protocol for the ENCODE trial: evaluating a novel online depression intervention for persons with epilepsy
Abstract
Background: Depression is common among persons with epilepsy (PwE), affecting roughly one in three individuals, and its presence is associated with personal suffering, impaired quality of life, and worse prognosis. Despite the availability of effective treatments, depression is often overlooked and treated inadequately in PwE, in part because of assumed concerns over drug interactions or proconvulsant effects of antidepressants. Internet-administered psychological interventions might complement antidepressant medication or psychotherapy, and preliminary evidence suggests that they can be effective. However, no trial has yet examined whether an Internet intervention designed to meet the needs of PwE can achieve sustained reductions in depression and related symptoms, such as anxiety, when offered as adjunct to treatment as usual.
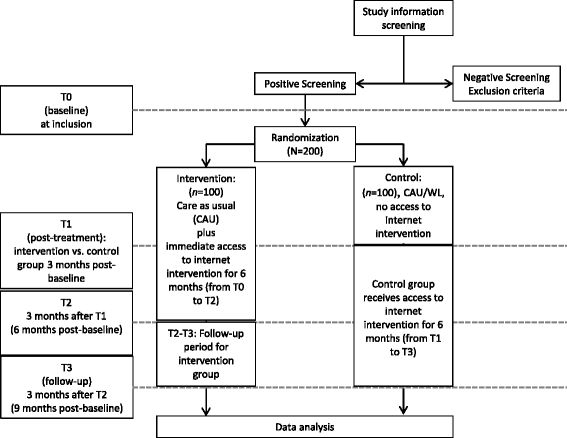
Methods/design: This randomized controlled trial will include 200 participants with epilepsy and a current depressive disorder, along with currently at least moderately elevated depression (Patient Health Questionnaire (PHQ-9) sum score of at least 10). Patients will be recruited via epilepsy treatment centers and other sources, including Internet forums, newspaper articles, flyers, posters, and media articles or advertisements, in German-speaking countries. Main inclusion criteria are: self-reported diagnosis of epilepsy and a depressive disorder, as assessed with a phone-administered structured diagnostic interview, none or stable antidepressant medication, no current psychotherapy, no other major psychiatric disorder, no acute suicidality. Participants will be randomly assigned to either (1) a care-as-usual/waitlist (CAU/WL) control group, in which they receive CAU and are given access to the Internet intervention after 3 months (that is, a CAU/WL control group), or (2) a treatment group that may also use CAU and in addition immediately receives six-month access to the novel, Internet-administered intervention. The primary outcome measure is the PHQ-9, collected at three months post-baseline; secondary measures include self-reported anxiety, work and social adjustment, epilepsy symptoms (including seizure frequency and severity), medication adherence, potential negative treatment effects and health-related quality of life. Measurements are collected online at pre-treatment (T0), three months (T1), six months (T2), and nine months (T3).
Discussion: Results of this trial are expected to extend the body of knowledge with regard to effective and efficient treatment options for PwE who experience elevated depression and anxiety.
Trial registration: ClinicalTrials.gov: NCT02791724 . Registered 01 June 2016.
Keywords: Anxiety; Depression; Epilepsy; Internet interventions.
Figures
Similar articles
-
Protocol for the Optimune trial: a randomized controlled trial evaluating a novel Internet intervention for breast cancer survivors.Trials. 2020 Jan 29;21(1):117. doi: 10.1186/s13063-019-3987-y. Trials. 2020. PMID: 31996235 Free PMC article.
-
Effects of an epilepsy-specific Internet intervention (Emyna) on depression: Results of the ENCODE randomized controlled trial.Epilepsia. 2019 Apr;60(4):656-668. doi: 10.1111/epi.14673. Epub 2019 Feb 25. Epilepsia. 2019. PMID: 30802941 Clinical Trial.
-
A randomized controlled trial of a therapist-guided online intervention for depressed adults and its utility as an adjunctive to antidepressants and psychotherapy.BMC Psychiatry. 2025 Feb 11;25(1):116. doi: 10.1186/s12888-025-06564-2. BMC Psychiatry. 2025. PMID: 39934725 Free PMC article. Clinical Trial.
-
Novel Augmentation Strategies in Major Depression.Dan Med J. 2017 Apr;64(4):B5338. Dan Med J. 2017. PMID: 28385173 Review.
-
The Generalizability of Randomized Controlled Trials of Self-Guided Internet-Based Cognitive Behavioral Therapy for Depressive Symptoms: Systematic Review and Meta-Regression Analysis.J Med Internet Res. 2018 Nov 9;20(11):e10113. doi: 10.2196/10113. J Med Internet Res. 2018. PMID: 30413400 Free PMC article.
Cited by
-
Neurology and the Internet: a review.Neurol Sci. 2018 Jun;39(6):981-987. doi: 10.1007/s10072-018-3339-9. Epub 2018 Mar 28. Neurol Sci. 2018. PMID: 29594831 Review.
-
Enhancing quality of life in epilepsy with a digital intervention (emyna): Results of the ELAINE randomized controlled trial.Epilepsia Open. 2024 Oct;9(5):1758-1771. doi: 10.1002/epi4.13014. Epub 2024 Aug 21. Epilepsia Open. 2024. PMID: 39167060 Free PMC article. Clinical Trial.
-
High Frequency of Depressive Symptoms among Adults with Epilepsy: Results from a Hospital-based Study.J Neurosci Rural Pract. 2017 Aug;8(Suppl 1):S13-S19. doi: 10.4103/jnrp.jnrp_21_17. J Neurosci Rural Pract. 2017. PMID: 28936065 Free PMC article.
-
Development of an epilepsy self-management mobile health app framework: Content validity study results.PLoS One. 2024 Jun 7;19(6):e0302844. doi: 10.1371/journal.pone.0302844. eCollection 2024. PLoS One. 2024. PMID: 38848353 Free PMC article.
-
Epilepsy and Depression: A Bidirectional Relationship.J Neurosci Rural Pract. 2017 Aug;8(Suppl 1):S5-S6. doi: 10.4103/jnrp.jnrp_203_17. J Neurosci Rural Pract. 2017. PMID: 28936063 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical