Marrow adipocytes inhibit the differentiation of mesenchymal stem cells into osteoblasts via suppressing BMP-signaling
- PMID: 28173811
- PMCID: PMC5296965
- DOI: 10.1186/s12929-017-0321-4
Marrow adipocytes inhibit the differentiation of mesenchymal stem cells into osteoblasts via suppressing BMP-signaling
Abstract
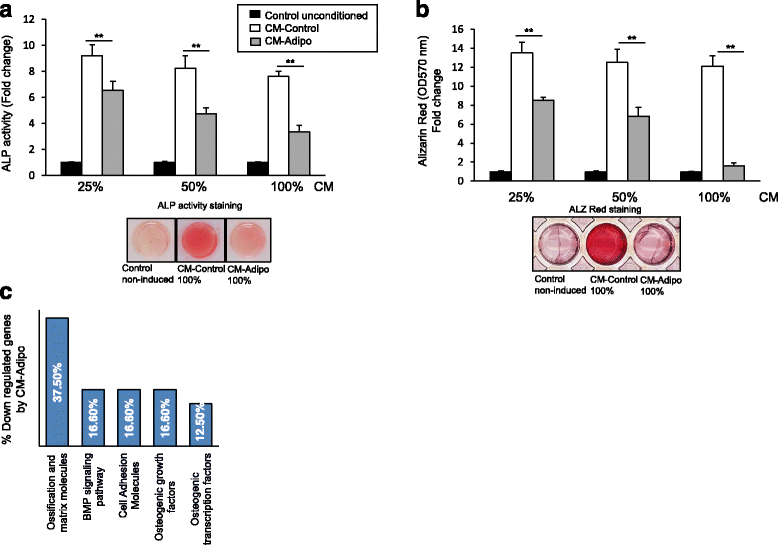
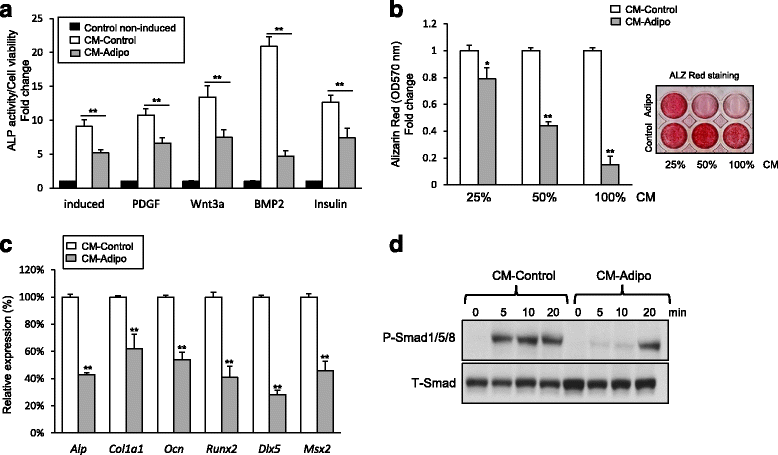
Background: Reduced bone formation is associated with increased bone marrow fat in many bone-loss related diseases including aging, post-menopause, and anorexia nervosa. Several lines of evidence suggested the regulation of osteogenesis and adipogenesis of the bone marrow-derived mesenchymal (skeletal) stem cells (BMSCs) by paracrine mediators. This study aimed to investigate the impact of adipocytes-secreted factors on the cell proliferation and osteoblast differentiation of BMSCs.
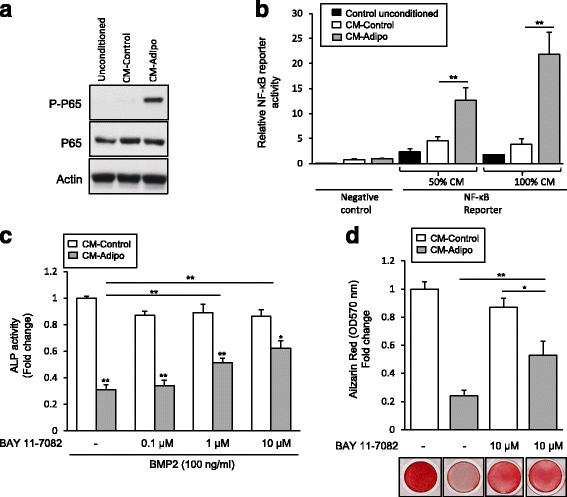
Methods: Serum free conditioned medium (CM-Adipo) was collected from stromal ST2 cells-derived adipocytes. Cell viability, quantitative alkaline phosphatase (ALP) activity assay, Alizarin red staining for matrix mineralization and osteogenic gene array expression were performed to determine the effect of CM-Adipo on cell proliferation and osteoblast differentiation of primary murine BMSCs (mBMSCs). Regulation of BMPs and NF-κB signaling pathways by CM-Adipo were detected by Western blot analysis and gene reporter assay.
Results: CM-Adipo showed no effect on cell viability/proliferation of primary mBMSCs as compared to CM-control. On the other hand, CM-Adipo significantly inhibited the commitment of mBMSCs into osteoblastic cell lineage in dose-dependent manner. CM-Adipo was found to dramatically inhibit the BMP2-induced osteoblast differentiation and to activate the inflammatory NF-κB signaling in mBMSCs. Interestingly, treatment of mBMSCs with the selective inhibitor of NF-κB pathway, BAY11-770682, showed to retrieve the inhibitory effect of CM-Adipo on BMP2-induced osteoblast differentiation in mBMSCs.
Conclusions: Our data demonstrated that the marrow adipocytes exert paracrine inhibitory effect on the osteoblast differentiation of mBMSCs by blocking BMPs signaling in a mechanism mediated by adipokines-induced NF-κB pathway activation.
Keywords: Adipocyte; BMSCs; Mesenchymal stem cells; Osteoblast; Paracrine factors; osteoblast differentiation.
Figures

Similar articles
-
5'-hydroxy Auraptene stimulates osteoblast differentiation of bone marrow-derived mesenchymal stem cells via a BMP-dependent mechanism.J Biomed Sci. 2019 Jul 5;26(1):51. doi: 10.1186/s12929-019-0544-7. J Biomed Sci. 2019. PMID: 31277646 Free PMC article.
-
Secreted Clusterin protein inhibits osteoblast differentiation of bone marrow mesenchymal stem cells by suppressing ERK1/2 signaling pathway.Bone. 2018 May;110:221-229. doi: 10.1016/j.bone.2018.02.018. Epub 2018 Feb 21. Bone. 2018. PMID: 29476977
-
Wnt signalling mediates the cross-talk between bone marrow derived pre-adipocytic and pre-osteoblastic cell populations.Exp Cell Res. 2011 Apr 1;317(6):745-56. doi: 10.1016/j.yexcr.2010.12.015. Epub 2011 Jan 4. Exp Cell Res. 2011. PMID: 21211534
-
Fate decision of mesenchymal stem cells: adipocytes or osteoblasts?Cell Death Differ. 2016 Jul;23(7):1128-39. doi: 10.1038/cdd.2015.168. Epub 2016 Feb 12. Cell Death Differ. 2016. PMID: 26868907 Free PMC article. Review.
-
Plasticity and regulation of human bone marrow stromal osteoprogenitor cells: potential implication in the treatment of age-related bone loss.Histol Histopathol. 2004 Jan;19(1):151-7. doi: 10.14670/HH-19.151. Histol Histopathol. 2004. PMID: 14702183 Review.
Cited by
-
Single-Cell RNA-Sequencing Reveals the Skeletal Cellular Dynamics in Bone Repair and Osteoporosis.Int J Mol Sci. 2023 Jun 6;24(12):9814. doi: 10.3390/ijms24129814. Int J Mol Sci. 2023. PMID: 37372962 Free PMC article. Review.
-
Osteometabolism: Metabolic Alterations in Bone Pathologies.Cells. 2022 Dec 6;11(23):3943. doi: 10.3390/cells11233943. Cells. 2022. PMID: 36497201 Free PMC article. Review.
-
Frequency-specific sensitivity of 3T3-L1 preadipocytes to low-intensity vibratory stimulus during adipogenesis.In Vitro Cell Dev Biol Anim. 2022 Jun;58(6):452-461. doi: 10.1007/s11626-022-00696-5. Epub 2022 Jun 17. In Vitro Cell Dev Biol Anim. 2022. PMID: 35713773
-
5'-hydroxy Auraptene stimulates osteoblast differentiation of bone marrow-derived mesenchymal stem cells via a BMP-dependent mechanism.J Biomed Sci. 2019 Jul 5;26(1):51. doi: 10.1186/s12929-019-0544-7. J Biomed Sci. 2019. PMID: 31277646 Free PMC article.
-
Carnosol induces the osteogenic differentiation of bone marrow-derived mesenchymal stem cells via activating BMP-signaling pathway.Korean J Physiol Pharmacol. 2021 May 1;25(3):197-206. doi: 10.4196/kjpp.2021.25.3.197. Korean J Physiol Pharmacol. 2021. PMID: 33859060 Free PMC article.
References
-
- Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–64. doi: 10.1016/S0092-8674(00)80258-5. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

