Immunoselected STRO-3+ mesenchymal precursor cells reduce inflammation and improve clinical outcomes in a large animal model of monoarthritis
- PMID: 28173831
- PMCID: PMC5297153
- DOI: 10.1186/s13287-016-0460-7
Immunoselected STRO-3+ mesenchymal precursor cells reduce inflammation and improve clinical outcomes in a large animal model of monoarthritis
Abstract
Background: The purpose of this study was to investigate the therapeutic efficacy of intravenously administered immunoselected STRO-3 + mesenchymal precursor cells (MPCs) on clinical scores, joint pathology and cytokine production in an ovine model of monoarthritis.
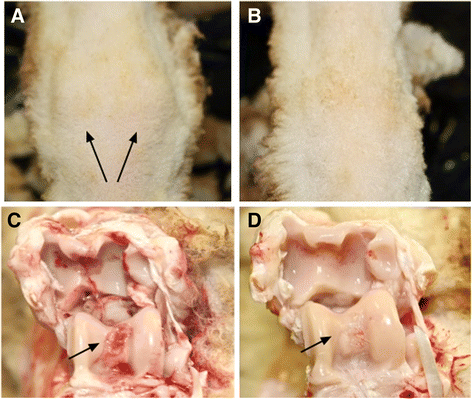
Methods: Monoarthritis was established in 16 adult merino sheep by administration of bovine type II collagen into the left hock joint following initial sensitization to this antigen. After 24 h, sheep were administered either 150 million allogeneic ovine MPCs (n = 8) or saline (n = 8) intravenously (IV). Lameness, joint swelling and pain were monitored and blood samples for leukocytes and cytokine levels were collected at intervals following arthritis induction. Animals were necropsied 14 days after arthritis induction and gross and histopathological evaluations were undertaken on tissues from the arthritic (left) and contralateral (right) joints.
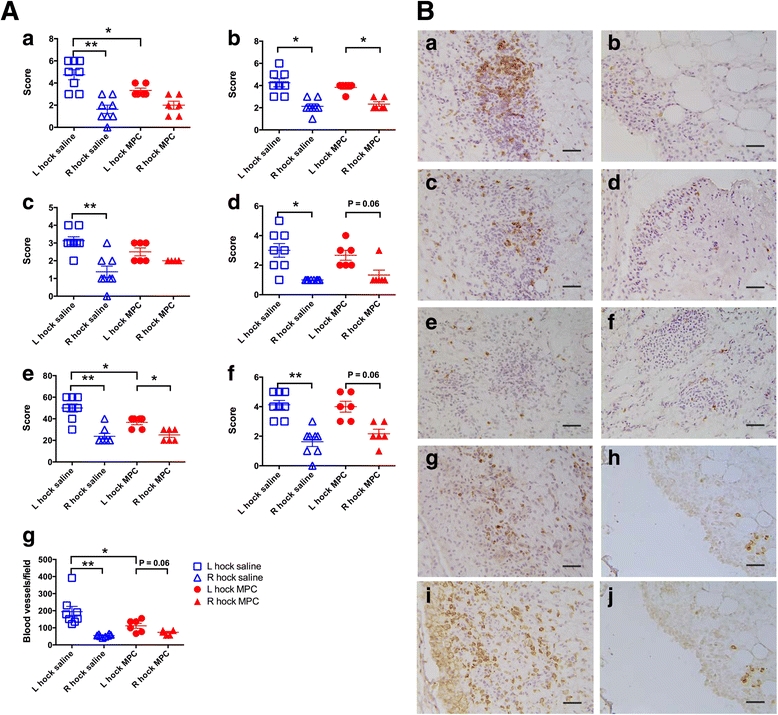
Results: MPC-treated sheep demonstrated significantly reduced clinical signs of lameness, joint pain and swelling compared with saline controls. They also showed decreased cartilage erosions, synovial stromal cell activation and angiogenesis. This was accompanied by decreased infiltration of the synovial tissues by CD4+ lymphocytes and CD14+ monocytes/macrophages. Over the 3 days following joint arthropathy induction, the numbers of neutrophils circulating in the blood and plasma concentrations of activin A were significantly reduced in animals administered MPCs.
Conclusions: The results of this study have demonstrated the capacity of IV-administered MPCs to mitigate the clinical signs and some of the inflammatory mediators responsible for joint tissue destruction in a large animal model of monoarthritis.
Keywords: Animal model; Collagen-induced arthritis; Mesenchymal stem cells; Neutrophils.
Figures






Similar articles
-
Effect of mesenchymal precursor cells on the systemic inflammatory response and endothelial dysfunction in an ovine model of collagen-induced arthritis.PLoS One. 2015 May 7;10(5):e0124144. doi: 10.1371/journal.pone.0124144. eCollection 2015. PLoS One. 2015. PMID: 25950840 Free PMC article.
-
Clinical and histopathological characterization of a large animal (ovine) model of collagen-induced arthritis.Vet Immunol Immunopathol. 2014 May 15;159(1-2):83-90. doi: 10.1016/j.vetimm.2014.03.007. Epub 2014 Mar 20. Vet Immunol Immunopathol. 2014. PMID: 24703062
-
Ovine synovial membrane-derived mesenchymal progenitor cells retain the phenotype of the original tissue that was exposed to in-vivo inflammation: evidence for a suppressed chondrogenic differentiation potential of the cells.Inflamm Res. 2012 Jun;61(6):599-608. doi: 10.1007/s00011-012-0450-x. Epub 2012 Mar 4. Inflamm Res. 2012. PMID: 22391623
-
Effector Functions of CD4+ T Cells at the Site of Local Autoimmune Inflammation-Lessons From Rheumatoid Arthritis.Front Immunol. 2019 Mar 12;10:353. doi: 10.3389/fimmu.2019.00353. eCollection 2019. Front Immunol. 2019. PMID: 30915067 Free PMC article. Review.
-
[The pathogenesis of joint destruction in chronic polyarthritis].Radiologe. 1996 Aug;36(8):593-9. doi: 10.1007/s001170050116. Radiologe. 1996. PMID: 8975275 Review. German.
Cited by
-
Unveiling heterogeneity in MSCs: exploring marker-based strategies for defining MSC subpopulations.J Transl Med. 2024 May 15;22(1):459. doi: 10.1186/s12967-024-05294-5. J Transl Med. 2024. PMID: 38750573 Free PMC article. Review.
-
Stem cell therapy for heart failure in the clinics: new perspectives in the era of precision medicine and artificial intelligence.Front Physiol. 2024 Jan 9;14:1344885. doi: 10.3389/fphys.2023.1344885. eCollection 2023. Front Physiol. 2024. PMID: 38264333 Free PMC article. Review.
-
Safety, tolerability, clinical, and joint structural outcomes of a single intra-articular injection of allogeneic mesenchymal precursor cells in patients following anterior cruciate ligament reconstruction: a controlled double-blind randomised trial.Arthritis Res Ther. 2017 Aug 2;19(1):180. doi: 10.1186/s13075-017-1391-0. Arthritis Res Ther. 2017. PMID: 28768528 Free PMC article. Clinical Trial.
-
Phase 3 DREAM-HF Trial of Mesenchymal Precursor Cells in Chronic Heart Failure.Circ Res. 2019 Jul 19;125(3):265-281. doi: 10.1161/CIRCRESAHA.119.314951. Epub 2019 Jul 18. Circ Res. 2019. PMID: 31318648 Free PMC article. Review.
-
The treatment of articular cartilage injuries with mesenchymal stem cells in different animal species.Open Vet J. 2021 Jan-Mar;11(1):128-134. doi: 10.4314/ovj.v11i1.19. Epub 2021 Feb 16. Open Vet J. 2021. PMID: 33898294 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

