Development of real-time and lateral flow strip reverse transcription recombinase polymerase Amplification assays for rapid detection of peste des petits ruminants virus
- PMID: 28173845
- PMCID: PMC5297045
- DOI: 10.1186/s12985-017-0688-6
Development of real-time and lateral flow strip reverse transcription recombinase polymerase Amplification assays for rapid detection of peste des petits ruminants virus
Abstract
Background: Peste des petits ruminants (PPR) is an economically important, Office International des Epizooties (OIE) notifiable, transboundary viral disease of small ruminants such as sheep and goat. PPR virus (PPRV), a negative-sense single-stranded RNA virus, is the causal agent of PPR. Therefore, sensitive, specific and rapid diagnostic assay for the detection of PPRV are necessary to accurately and promptly diagnose suspected case of PPR.
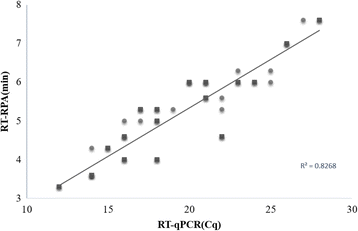
Methods: In this study, reverse transcription recombinase polymerase amplification assays using real-time fluorescent detection (real-time RT-RPA assay) and lateral flow strip detection (LFS RT-RPA assay) were developed targeting the N gene of PPRV.
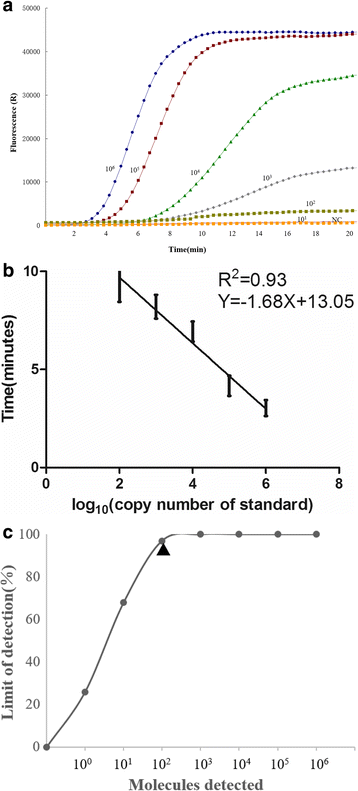
Results: The sensitivity of the developed real-time RT-RPA assay was as low as 100 copies per reaction within 7 min at 40 °C with 95% reliability; while the sensitivity of the developed LFS RT-RPA assay was as low as 150 copies per reaction at 39 °C in less than 25 min. In both assays, there were no cross-reactions with sheep and goat pox viruses, foot-and-mouth disease virus and Orf virus.
Conclusions: These features make RPA assay promising candidates either in field use or as a point of care diagnostic technique.
Keywords: Lateral flow strip; PPRV; RT-RPA; Reverse transcription recombinase polymerase amplification assay; Small ruminants.
Figures



Similar articles
-
Rapid detection of peste des petits ruminants virus by a reverse transcription loop-mediated isothermal amplification assay.J Virol Methods. 2010 Dec;170(1-2):37-41. doi: 10.1016/j.jviromet.2010.08.016. Epub 2010 Sep 9. J Virol Methods. 2010. PMID: 20813134
-
Development of real-time reverse transcription recombinase polymerase amplification (RPA) for rapid detection of peste des petits ruminants virus in clinical samples and its comparison with real-time PCR test.Sci Rep. 2018 Dec 10;8(1):17760. doi: 10.1038/s41598-018-35636-5. Sci Rep. 2018. PMID: 30531986 Free PMC article.
-
Development of recombinase polymerase amplification assays for the rapid detection of peste des petits ruminants virus.J Virol Methods. 2018 Apr;254:35-39. doi: 10.1016/j.jviromet.2018.01.012. Epub 2018 Feb 2. J Virol Methods. 2018. PMID: 29407212
-
Peste des petits ruminants in Africa: a review of currently available molecular epidemiological data, 2020.Arch Virol. 2020 Oct;165(10):2147-2163. doi: 10.1007/s00705-020-04732-1. Epub 2020 Jul 11. Arch Virol. 2020. PMID: 32653984 Free PMC article. Review.
-
Comparative efficacy of peste des petits ruminants (PPR) vaccines.Biologicals. 2010 Jul;38(4):479-85. doi: 10.1016/j.biologicals.2010.02.003. Epub 2010 Mar 2. Biologicals. 2010. PMID: 20199873 Review.
Cited by
-
Rapid isothermal duplex real-time recombinase polymerase amplification (RPA) assay for the diagnosis of equine piroplasmosis.Sci Rep. 2020 Mar 5;10(1):4096. doi: 10.1038/s41598-020-60997-1. Sci Rep. 2020. PMID: 32139744 Free PMC article.
-
Simultaneous detection and identification of Peste des petits ruminants Virus Lineages II and IV by MCA-Based real-time quantitative RT-PCR assay within single reaction.BMC Vet Res. 2023 Jan 16;19(1):11. doi: 10.1186/s12917-023-03568-6. BMC Vet Res. 2023. PMID: 36647038 Free PMC article.
-
Establishment of a recombinase polymerase amplification (RPA) assay for the detection of Brucella spp. Infection.Mol Cell Probes. 2019 Oct;47:101434. doi: 10.1016/j.mcp.2019.101434. Epub 2019 Aug 8. Mol Cell Probes. 2019. PMID: 31401295 Free PMC article.
-
Point-of-care diagnostic assay for rapid detection of porcine deltacoronavirus using the recombinase polymerase amplification method.Transbound Emerg Dis. 2019 May;66(3):1324-1331. doi: 10.1111/tbed.13155. Epub 2019 Apr 1. Transbound Emerg Dis. 2019. PMID: 30801935 Free PMC article.
-
Recombinase polymerase amplification assay for the detection of piper yellow mottle virus infecting black pepper.Virusdisease. 2020 Mar;31(1):38-44. doi: 10.1007/s13337-019-00566-x. Epub 2020 Jan 27. Virusdisease. 2020. PMID: 32206697 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

