Therapeutic efficacy and effects of artemisinin-based combination treatments on uncomplicated Plasmodium falciparum malaria -associated anaemia in Nigerian children during seven years of adoption as first-line treatments
- PMID: 28173853
- PMCID: PMC5294876
- DOI: 10.1186/s40249-016-0217-7
Therapeutic efficacy and effects of artemisinin-based combination treatments on uncomplicated Plasmodium falciparum malaria -associated anaemia in Nigerian children during seven years of adoption as first-line treatments
Abstract
Background: Artemisinin-based combination treatments (ACTs) are the first-line treatments of uncomplicated Plasmodium falciparum malaria in many endemic areas but there are few evaluation of their efficacy in anaemic malarious children.
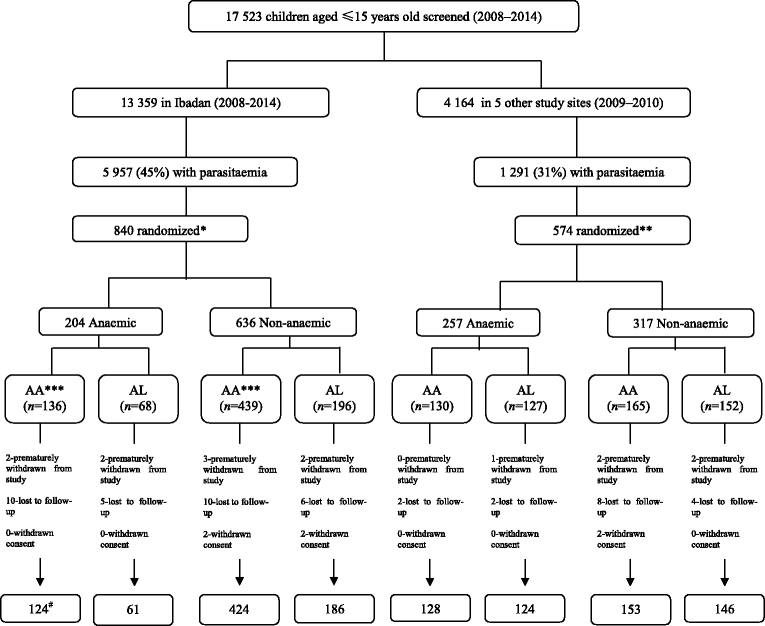
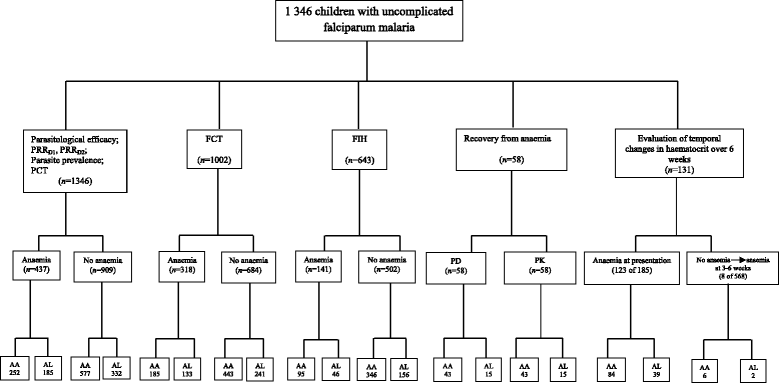
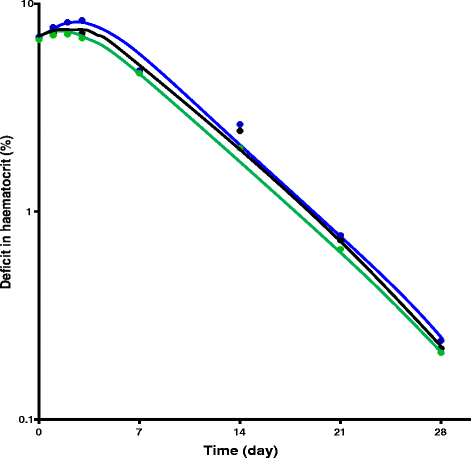
Methods: Therapeutic efficacy of 3-day regimens of artesunate-amodiaquine and artemether-lumefantrine was evaluated in 437 anaemic and 909 non-anaemic malarious children following treatment during a seven-year period (2008-2014). Patterns of temporal changes in haematocrit were classified based on haematocrit values <30% and ≥30%. Kinetics of the disposition of the deficit in haematocrit from 30% following treatment were evaluated using a non-compartment model.
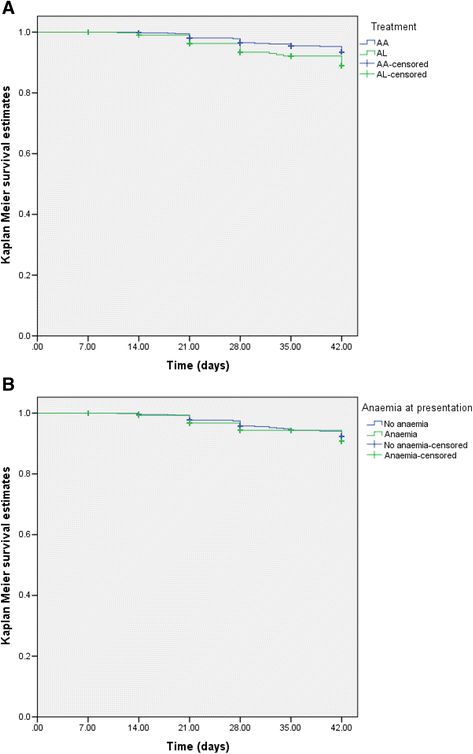
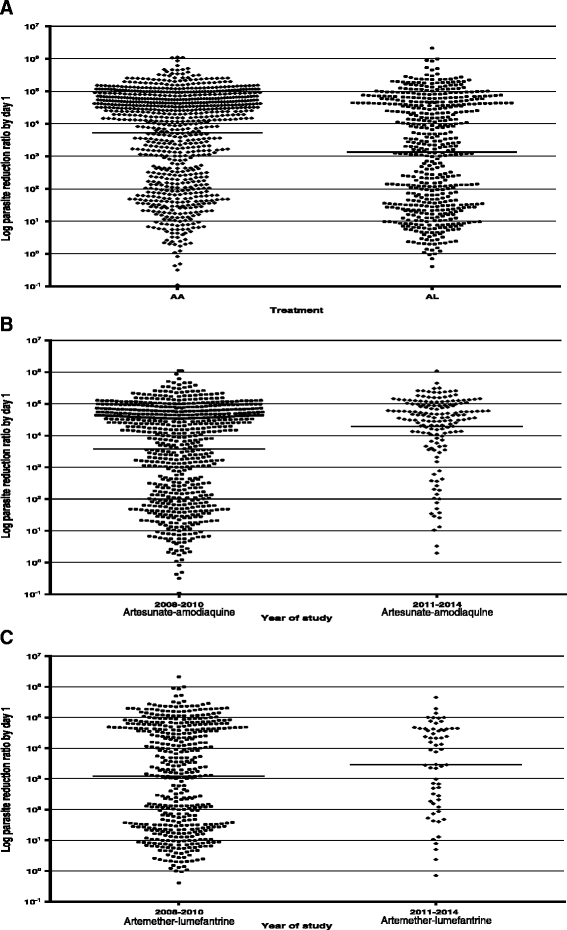
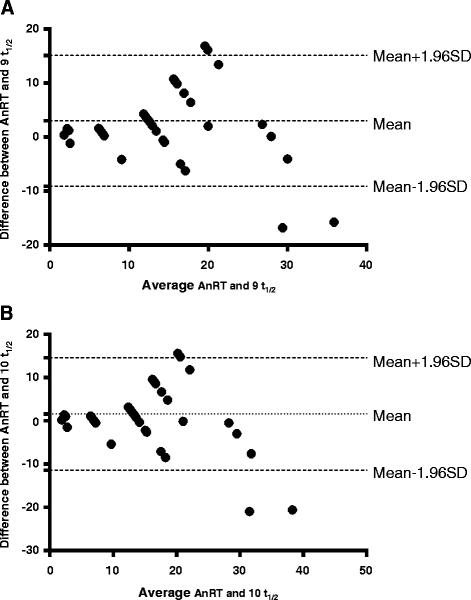
Results: PCR-corrected parasitological efficacy 28 days after start of treatment was significantly higher in artesunate-amodiaquine- compared to artemether-lumefantrine-treated children [97% (95%CI: 92.8-100) versus 96.4% (95%CI: 91.3-99.4), P = 0.02], but it was similar in non-anaemic and anaemic children. Fall in haematocrit/1 000 asexual parasites cleared from peripheral blood was significantly greater at lower compared to higher parasitaemias (P < 0.0001), and in non-anaemic compared to anaemic children (P = 0.007). In anaemic children at presentation, mean anaemia recovery time (AnRT) was 15.4 days (95%CI: 13.3-17.4) and it did not change over the years. Declines in haematocrit deficits from 30% were monoexponential with mean estimated half-time of 1.4 days (95%CI: 1.2-1.6). Anaemia half-time (t½anaemia) correlated positively with AnRT in the same patients (r = 0.69, P < 0.0001). Bland-Altman analysis of 10 multiples of t½anaemia and AnRT showed narrow limit of agreement with insignificant bias (P = 0.07) suggesting both can be used interchangeably in the same patients.
Conclusions: Artesunate-amodiaquine and artemether-lumefantrine remain efficacious treatments of uncomplicated P. falciparum infections in non-anaemic and anaemic Nigerian children in the last 7 years of adoption as first-line treatments. These ACTs may also conserve haematocrit at high parasitaemias and in anaemic children.
Trials registration: Pan African Clinical Trial Registry PACTR201508001188143 , 3 July 2015; PACTR201510001189370 , 3 July 2015; PACTR201508001191898 , 7 July 2015 and PACTR201508001193368 , 8 July 2015.
Keywords: Artemisinin-based combination treatments; Children; Malaria-associated anaemia; Nigeria; “Haematocrit conservation”.
Figures
Similar articles
-
Factors contributing to anaemia after uncomplicated falciparum malaria in under five year-old Nigerian children ten years following adoption of artemisinin-based combination therapies as first-line antimalarials.BMC Infect Dis. 2017 Dec 19;17(1):781. doi: 10.1186/s12879-017-2876-9. BMC Infect Dis. 2017. PMID: 29258448 Free PMC article.
-
Therapeutic efficacy and effects of artesunate-amodiaquine and artemether-lumefantrine on malaria-associated anaemia in Nigerian children aged two years and under.Infect Dis Poverty. 2016 Jul 6;5(1):70. doi: 10.1186/s40249-016-0165-2. Infect Dis Poverty. 2016. PMID: 27384596 Free PMC article.
-
Temporal changes in haematocrit following artemisinin-based combination treatments of uncomplicated falciparum malaria in children.BMC Infect Dis. 2015 Oct 26;15:454. doi: 10.1186/s12879-015-1219-y. BMC Infect Dis. 2015. PMID: 26502714 Free PMC article. Clinical Trial.
-
Pyronaridine-artesunate for treating uncomplicated Plasmodium falciparum malaria.Cochrane Database Syst Rev. 2019 Jan 8;1(1):CD006404. doi: 10.1002/14651858.CD006404.pub3. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2022 Jun 21;6:CD006404. doi: 10.1002/14651858.CD006404.pub4. PMID: 30620055 Free PMC article. Updated.
-
Atovaquone-proguanil for treating uncomplicated Plasmodium falciparum malaria.Cochrane Database Syst Rev. 2021 Jan 15;1(1):CD004529. doi: 10.1002/14651858.CD004529.pub3. Cochrane Database Syst Rev. 2021. PMID: 33459345 Free PMC article.
Cited by
-
Parasite reduction ratio one day after initiation of artemisinin-based combination therapies and its relationship with parasite clearance time in acutely malarious children.Infect Dis Poverty. 2018 Dec 7;7(1):122. doi: 10.1186/s40249-018-0503-7. Infect Dis Poverty. 2018. PMID: 30522524 Free PMC article. Clinical Trial.
-
Factors contributing to anaemia after uncomplicated falciparum malaria in under five year-old Nigerian children ten years following adoption of artemisinin-based combination therapies as first-line antimalarials.BMC Infect Dis. 2017 Dec 19;17(1):781. doi: 10.1186/s12879-017-2876-9. BMC Infect Dis. 2017. PMID: 29258448 Free PMC article.
-
Anti-malarial activity of traditional Kampo medicine Coptis rhizome extract and its major active compounds.Malar J. 2020 Jun 8;19(1):204. doi: 10.1186/s12936-020-03273-x. Malar J. 2020. PMID: 32513250 Free PMC article.
-
Therapeutic Efficacy of Artemether-Lumefantrine (Coartem®) for the Treatment of Uncomplicated Falciparum Malaria in Africa: A Systematic Review.J Parasitol Res. 2020 Oct 20;2020:7371681. doi: 10.1155/2020/7371681. eCollection 2020. J Parasitol Res. 2020. PMID: 33145101 Free PMC article. Review.
-
Solidification of Self-Emulsifying Drug Delivery Systems as a Novel Approach to the Management of Uncomplicated Malaria.Pharmaceuticals (Basel). 2022 Jan 20;15(2):120. doi: 10.3390/ph15020120. Pharmaceuticals (Basel). 2022. PMID: 35215233 Free PMC article. Review.
References
-
- World Health Organization . Antimalarial Drug Combination Therapy. Report of a WHO Technical Consultation. Geneva: World Health Organization; 2001.
-
- Noedl H, Se Y, Sriwichai S, Schaecher K, Teja-Isavadharm P, Smith B, Rutvisuttinunt W, Bethell D, Surasri S, Fukuda MM, Socheat D, Cham TL. Artemisinin resistance in Cambodia: a clinical trial designed to address an emerging problem in Southeast Asia. Clin Infect Dis. 2010;51:e82–e89. doi: 10.1086/657120. - DOI - PubMed
-
- Carrara VI, Zwang J, Ashley EA, Price RN, Stepniewka K, Marion B, Brockman A, Anderson T, McGready R, Phaiphun L, Stephane P, van Vugt M, Hutagalung R, Lwin KM, Phyo AP, Piyanuch P, Imwong M, Pukrittayakamee S, Singhasivanon P, White NJ, Nosten F. Changes in the treatment responses to artesunate-mefloquine on the northwestern border of Thailand during 13 years of continuous deployment. PLoS One. 2009;4:e511. doi: 10.1371/journal.pone.0004551. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

