Anti-influenza virus activity of extracts from the stems of Jatropha multifida Linn. collected in Myanmar
- PMID: 28173854
- PMCID: PMC5297253
- DOI: 10.1186/s12906-017-1612-8
Anti-influenza virus activity of extracts from the stems of Jatropha multifida Linn. collected in Myanmar
Abstract
Background: To contribute to the development of novel anti-influenza drugs, we investigated the anti-influenza activity of crude extracts from 118 medicinal plants collected in Myanmar. We discovered that extract from the stems of Jatropha multifida Linn. showed anti-influenza activity. J. multifida has been used in traditional medicine for the treatment of various diseases, and the stem has been reported to possess antimicrobial, antimalarial, and antitumor activities. However, the anti-influenza activity of this extract has not yet been investigated.
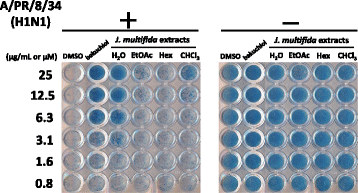
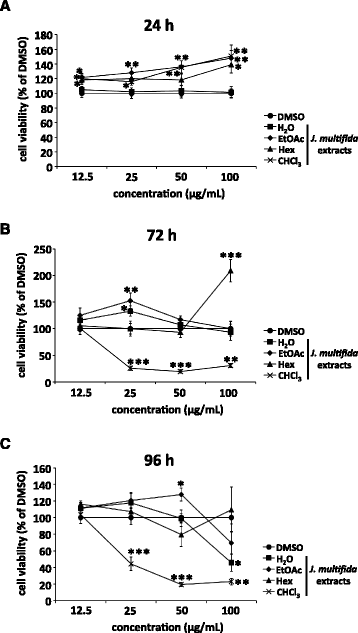
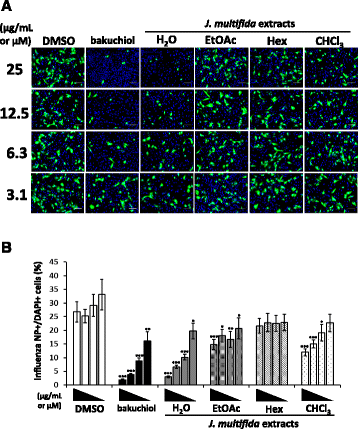
Methods: We prepared water (H2O), ethyl acetate (EtOAc), n-hexane (Hex), and chloroform (CHCl3) extracts from the stems of J. multifida collected in Myanmar, and examined the survival of Madin-Darby canine kidney (MDCK) cells infected with the influenza A (H1N1) virus, and the inhibitory effects of these crude extracts on influenza A viral infection and growth in MDCK cells.
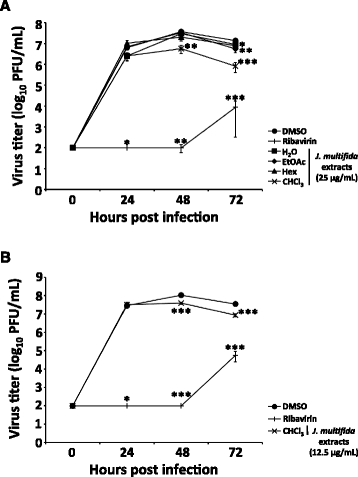
Results: The H2O extracts from the stems of J. multifida promoted the survival of MDCK cells infected with the influenza A H1N1 virus. The EtOAc and CHCl3 extracts resulted in similar, but weaker, effects. The H2O, EtOAc, and CHCl3 extracts from the stems of J. multifida inhibited influenza A virus H1N1 infection; the H2O extract possessed the strongest inhibitory effect on influenza infection in MDCK cells. The EtOAc, Hex, and CHCl3 extracts all inhibited the growth of influenza A H1N1 virus, and the CHCl3 extract demonstrated the strongest activity in MDCK cells.
Conclusion: The H2O or CHCl3 extracts from the stems of J. multifida collected in Myanmar demonstrated the strongest inhibition of influenza A H1N1 viral infection or growth in MDCK cells, respectively. These results indicated that the stems of J. multifida could be regarded as an anti-influenza herbal medicine as well as a potential crude drug source for the development of anti-influenza compounds.
Keywords: Anti-influenza; Anti-virus; Herbal medicine; Jatropha multifida; Stem.
Figures
Similar articles
-
Antiviral activity of hydroalcoholic extract from Eupatorium perfoliatum L. against the attachment of influenza A virus.J Ethnopharmacol. 2016 Jul 21;188:144-52. doi: 10.1016/j.jep.2016.05.016. Epub 2016 May 10. J Ethnopharmacol. 2016. PMID: 27178637
-
Anti-influenza virus effects of cocoa.J Sci Food Agric. 2016 Mar 15;96(4):1150-8. doi: 10.1002/jsfa.7197. Epub 2015 Apr 27. J Sci Food Agric. 2016. PMID: 25847473 Clinical Trial.
-
Anti-Influenza Activity of an Ethyl Acetate Fraction of a Rhus verniciflua Ethanol Extract by Neuraminidase Inhibition.Oxid Med Cell Longev. 2020 Oct 29;2020:8824934. doi: 10.1155/2020/8824934. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 33204399 Free PMC article.
-
Synthesis of pterodontic acid derivatives and the study of their anti-influenza A virus (H1N1) activity.Fitoterapia. 2021 Jul;152:104942. doi: 10.1016/j.fitote.2021.104942. Epub 2021 May 21. Fitoterapia. 2021. PMID: 34029655 Review.
-
Biological activities: anti-infectious, antioxidant and healing of the vegetable species Jatropha multifida.Rev Bras Enferm. 2021 May 28;74(2):e20200451. doi: 10.1590/0034-7167-2020-0451. eCollection 2021. Rev Bras Enferm. 2021. PMID: 34076218 Review. English, Portuguese.
Cited by
-
Inactivation of plant and animal viruses by proanthocyanidins from Alpinia zerumbet extract.Plant Biotechnol (Tokyo). 2021 Dec 25;38(4):453-455. doi: 10.5511/plantbiotechnology.21.0925a. Plant Biotechnol (Tokyo). 2021. PMID: 35087311 Free PMC article.
-
Antiviral Activities of Mulberry (Morus alba) Juice and Seed against Influenza Viruses.Evid Based Complement Alternat Med. 2018 Nov 1;2018:2606583. doi: 10.1155/2018/2606583. eCollection 2018. Evid Based Complement Alternat Med. 2018. PMID: 30515232 Free PMC article.
-
Effects of Plant Metabolites on the Growth of COVID-19 (Coronavirus Disease-19) Including Omicron Strain.Cureus. 2022 Jul 4;14(7):e26549. doi: 10.7759/cureus.26549. eCollection 2022 Jul. Cureus. 2022. PMID: 35936126 Free PMC article. Review.
-
Bioactive Natural Antivirals: An Updated Review of the Available Plants and Isolated Molecules.Molecules. 2020 Oct 22;25(21):4878. doi: 10.3390/molecules25214878. Molecules. 2020. PMID: 33105694 Free PMC article. Review.
-
Medicinal plants: Treasure for antiviral drug discovery.Phytother Res. 2021 Jul;35(7):3447-3483. doi: 10.1002/ptr.7039. Epub 2021 Feb 16. Phytother Res. 2021. PMID: 33590931 Free PMC article. Review.
References
-
- Fiore AE, Fry A, Shay D, Gubareva L, Bresee JS, Uyeki TM. Centers for Disease C, Prevention: Antiviral agents for the treatment and chemoprophylaxis of influenza --- recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2011;60(1):1–24. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

