Characteristics of human adipose derived stem cells in scleroderma in comparison to sex and age matched normal controls: implications for regenerative medicine
- PMID: 28173869
- PMCID: PMC5297142
- DOI: 10.1186/s13287-016-0444-7
Characteristics of human adipose derived stem cells in scleroderma in comparison to sex and age matched normal controls: implications for regenerative medicine
Abstract
Background: Adipose-derived stem cells (ADSCs) are emerging as an alternative stem cell source for cell-based therapies. Recent data suggest that autologous ADSC-enriched micrografting improves the effects of facial involvement in systemic sclerosis (SSc). We have extensively characterised ADSCs from SSc patients and compared their phenotype and function to healthy age- and sex-matched control ADSCs.
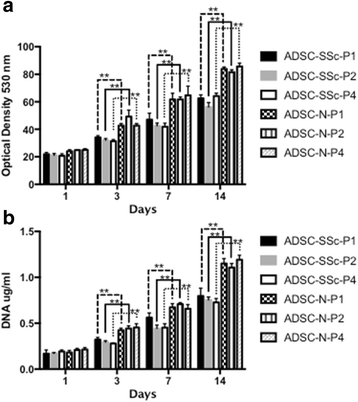
Methods: ADSCs were isolated and characterised from a cohort of six SSc patients (ADSC-SSc) and were compared to six healthy age- and sex-matched controls (ADSC-N). Cell surface phenotype lineage commitment was explored by flow cytometric analysis of mesenchymal and hematopoietic markers and by the capacity to differentiate to chondrogenic, osteogenic, and adipogenic lineages. Functional activities of ADSCs were assessed by biochemical and cellular assays for proliferation, metabolism, adhesion, morphology, migration, and invasion.
Results: Upon characterization of ADSC-SSc, we found that there was no alteration in the phenotype or surface antigen expression compared to healthy matched control ADSCs. We found that the differentiation capacity of ADSC-SSc was equivalent to that of ADSC-N, and that ADSC-SSc did not display any morphological or adhesive abnormalities. We found that the proliferation rate and metabolic activity of ADSC-SSc was reduced (p < 0.01). We found that the migration and invasion capacity of ADSC-SSc was reduced (p < 0.01) compared to healthy matched control ADSCs.
Conclusions: This study provides important findings that can differentially characterise ADSCs from SSc patients. Results indicate that the surface phenotype and differentiation capacity of ADSCs from SSc patients are identical to healthy matched ADSCs. While the findings indicate that the proliferation and migration capacity of ADSC-SSc is reduced, ADSC-SSc are capable of ex-vivo culture and expansion. These findings encourage further investigation into the understanding by which ADSCs can impact upon tissue fibrosis.
Keywords: Adipose-derived stem cells; Invasion; Metabolism; Migration; Proliferation; Regenerative medicine; Scleroderma; Systemic sclerosis.
Figures





Similar articles
-
Phenotypical and Functional Characteristics of In Vitro-Expanded Adipose-Derived Mesenchymal Stromal Cells From Patients With Systematic Sclerosis.Cell Transplant. 2017 May 9;26(5):841-854. doi: 10.3727/096368917X694822. Epub 2017 Jan 31. Cell Transplant. 2017. PMID: 28139194 Free PMC article.
-
Combined platelet-rich plasma and lipofilling treatment provides great improvement in facial skin-induced lesion regeneration for scleroderma patients.Stem Cell Res Ther. 2017 Oct 23;8(1):236. doi: 10.1186/s13287-017-0690-3. Stem Cell Res Ther. 2017. PMID: 29058626 Free PMC article.
-
Hypoxia Pretreatment Promotes Chondrocyte Differentiation of Human Adipose-Derived Stem Cells via Vascular Endothelial Growth Factor.Tissue Eng Regen Med. 2020 Jun;17(3):335-350. doi: 10.1007/s13770-020-00265-5. Epub 2020 May 26. Tissue Eng Regen Med. 2020. PMID: 32451775 Free PMC article.
-
Adipose-derived stem cells: Pathophysiologic implications vs therapeutic potential in systemic sclerosis.World J Stem Cells. 2021 Jan 26;13(1):30-48. doi: 10.4252/wjsc.v13.i1.30. World J Stem Cells. 2021. PMID: 33584978 Free PMC article. Review.
-
Adipose derived stem cells - Sources, differentiation capacity and a new target for reconstructive and regenerative medicine.Biomed Pharmacother. 2025 May;186:118036. doi: 10.1016/j.biopha.2025.118036. Epub 2025 Apr 6. Biomed Pharmacother. 2025. PMID: 40194335 Review.
Cited by
-
Unraveling the Pathogenesis of Calcinosis in Systemic Sclerosis: A Molecular and Clinical Insight.Int J Mol Sci. 2024 Oct 19;25(20):11257. doi: 10.3390/ijms252011257. Int J Mol Sci. 2024. PMID: 39457038 Free PMC article. Review.
-
The Influence of Negative Pressure and of the Harvesting Site on the Characteristics of Human Adipose Tissue-Derived Stromal Cells from Lipoaspirates.Stem Cells Int. 2020 Feb 10;2020:1016231. doi: 10.1155/2020/1016231. eCollection 2020. Stem Cells Int. 2020. PMID: 32104182 Free PMC article.
-
Administration Strategy-Dependent Mechanisms and Effects of Human Adipose Tissue Stem Cell Extracellular Vesicles in Mouse Allergic Rhinitis Treatment.Cell Transplant. 2025 Jan-Dec;34:9636897251325673. doi: 10.1177/09636897251325673. Epub 2025 Apr 3. Cell Transplant. 2025. PMID: 40179013 Free PMC article.
-
Stem cell enriched lipotransfer reverses the effects of fibrosis in systemic sclerosis.PLoS One. 2019 Jul 17;14(7):e0218068. doi: 10.1371/journal.pone.0218068. eCollection 2019. PLoS One. 2019. PMID: 31314805 Free PMC article. Clinical Trial.
-
Oro-facial fibrosis in systemic sclerosis: a reconstructive journey.BMJ Case Rep. 2020 Oct 10;13(10):e236663. doi: 10.1136/bcr-2020-236663. BMJ Case Rep. 2020. PMID: 33040038 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

