New insights into thyroid hormone action
- PMID: 28174093
- PMCID: PMC5407910
- DOI: 10.1016/j.pharmthera.2017.02.012
New insights into thyroid hormone action
Abstract
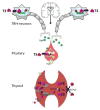
Thyroid hormones (TH) are endocrine messengers essential for normal development and function of virtually every vertebrate. The hypothalamic-pituitary-thyroid axis is exquisitely modulated to maintain nearly constant TH (T4 and T3) levels in circulation. However peripheral tissues and the CNS control the intracellular availability of TH, suggesting that circulating concentrations of TH are not fully representative of what each cell type sees. Indeed, recent work in the field has identified that TH transporters, deiodinases and thyroid hormone receptor coregulators can strongly control tissue-specific sensitivity to a set amount of TH. Furthermore, the mechanism by which the thyroid hormone receptors regulate target gene expression can vary by gene, tissue and cellular context. This review will highlight novel insights into the machinery that controls the cellular response to TH, which include unique signaling cascades. These findings shed new light into the pathophysiology of human diseases caused by abnormal TH signaling.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

