Epigenetic basis of the dark side of alcohol addiction
- PMID: 28174112
- PMCID: PMC5479721
- DOI: 10.1016/j.neuropharm.2017.02.002
Epigenetic basis of the dark side of alcohol addiction
Abstract
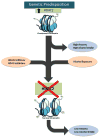
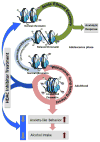
Alcoholism is a complex brain disease characterized by three distinct stages of the addiction cycle that manifest as neuroadaptive changes in the brain. One such stage of the addiction cycle is alcohol withdrawal and the negative affective states that promote drinking and maintain addiction. Repeated alcohol use, genetic predisposition to alcoholism and anxiety, and alcohol exposure during crucial developmental periods all contribute to the development of alcohol-induced withdrawal and negative affective symptoms. Epigenetic modifications within the amygdala have provided a molecular basis of these negative affective symptoms, also known as the dark side of addiction. Here, we propose that allostatic change within the epigenome in the amygdala is a prime mechanism of the biological basis of negative affective states resulting from, and contributing to, alcoholism. Acute alcohol exposure produces an anxiolytic response which is associated with the opening of chromatin due to increased histone acetylation, increased CREB binding protein (CBP) levels, and histone deacetylase (HDAC) inhibition. After chronic ethanol exposure, these changes return to baseline along with anxiety-like behaviors. However, during withdrawal, histone acetylation decreases due to increased HDAC activity and decreased CBP levels in the amygdala circuitry leading to the development of anxiety-like behaviors. Additionally, innately higher expression of the HDAC2 isoform leads to a deficit in global and gene-specific histone acetylation in the amygdala that is associated with a decrease in the expression of several synaptic plasticity-associated genes and maintaining heightened anxiety-like behavior and excessive alcohol intake. Adolescent alcohol exposure also leads to higher expression of HDAC2 and a deficit in histone acetylation leading to decreased expression of synaptic plasticity-associated genes and high anxiety and drinking behavior in adulthood. All these studies indicate that the epigenome can undergo allostatic reprogramming in the amygdaloid circuitry during various stages of alcohol exposure. Furthermore, opening the chromatin by inhibiting HDACs using pharmacological or genetic manipulations can lead to the attenuation of anxiety as well as alcohol intake. Chromatin remodeling provides a clear biological basis for the negative affective states seen during alcohol addiction and presents opportunities for novel drug development and treatment options. This article is part of the Special Issue entitled "Alcoholism".
Keywords: Addiction; Alcoholism; Anxiety; Dark side; Epigenetics; Gene expression.
Published by Elsevier Ltd.
Conflict of interest statement
SCP reports that a US patent application entitled “Histone acetyltransferase activators and histone deacetylase inhibitors in the treatment of alcoholism” (serial number 60/848237 filed on September 29th , 2006) is currently pending. EJ and HZ reported no potential conflicts of interest.
Figures

References
-
- Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, Vilpoux C, Naassila M. Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology. 2013;67:521–531. - PubMed
-
- Alheid GF. Extended amygdala and basal forebrain. Ann N Y Acad Sci. 2003;985:185–205. - PubMed
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5), Diagnostic and Statistical Manual of Mental Disorders 4th edition TR. 2013.
-
- Baldwin HA, Rassnick S, Rivier J, Koob GF, Britton KT. CRF antagonist reverses the “anxiogenic” response to ethanol withdrawal in the rat. Psychopharmacology (Berl) 1991;103:227–232. - PubMed
-
- Bannister A, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

