Contribution of industry funded post-marketing studies to drug safety: survey of notifications submitted to regulatory agencies
- PMID: 28174182
- PMCID: PMC5477378
- DOI: 10.1136/bmj.j337
Contribution of industry funded post-marketing studies to drug safety: survey of notifications submitted to regulatory agencies
Abstract
Objectives: To investigate the practice of post-marketing studies in Germany during a three year period and to evaluate whether these trials meet the aims specified in the German Medicinal Products Act.
Design: Survey of notifications submitted to German regulatory agencies before post-marketing studies were carried out, 2008-10.
Setting: Notifications obtained through freedom of information requests to the three authorities responsible for registering post-marketing studies in Germany.
Main outcome measures: Descriptive statistics of post-marketing studies, including the products under study, intended number of patients, intended number of participating physicians, proposed remunerations, study plan and protocol, and availability of associated scientific publications and reports on adverse drug reactions.
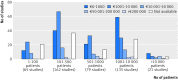
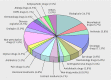
Results: Information was obtained from 558 studies, with a median of 600 (mean 2331, range 2-75 000) patients and 63 (270, 0-7000) participating physicians per study. The median remuneration to physicians per patient was €200 (€441, €0-€7280) (£170, £0-£6200; $215, $0-$7820), with a total remuneration cost of more than €217m for 558 studies registered over the three year period. The median remuneration per participating physician per study was €2000 (mean €19 424), ranging from €0 to €2 080 000. There was a broad range of drugs and non-drug products, of which only a third represented recently approved drugs. In many notifications, data, information, and results were, by contract, strictly confidential and the sole property of the respective sponsor. No single adverse drug reaction report could be identified from any of the 558 post-marketing studies. Less than 1% of studies could be verified as published in scientific journals.
Conclusions: Post-marketing studies are not improving drug safety surveillance. Sample sizes are generally too small to allow the detection of rare adverse drug reactions, and many participating physicians are strictly obliged to maintain confidentiality towards the sponsor. High remuneration and strict confidentiality clauses in these studies could influence the physicians' reporting behaviours of adverse drug reactions.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures

Comment in
-
Three design aspects for high quality post-marketing cohort studies.BMJ. 2017 Apr 12;357:j1851. doi: 10.1136/bmj.j1851. BMJ. 2017. PMID: 28404570 No abstract available.
-
Authors' reply to Fralick and Kesselheim.BMJ. 2017 Apr 12;357:j1850. doi: 10.1136/bmj.j1850. BMJ. 2017. PMID: 28404584 No abstract available.
References
-
- Council for International Organizations of Medical Sciences (CIOMS). Reporting Adverse Drug Reactions. Definitions of Terms and Criteria for their Use, CIOMS 1999. http://www.cioms.ch/publications/reporting_adverse_drug.pdf.
-
- German Medicinal Products Act (AMG), section 4, subsection 23, version 2004. Bundesgesetzesblatt 2004;I:2031.
-
- McCully S. Conducting non-interventional studies in Europe. Attempts at Clarity lead to increased complexity. http://www.inventivhealthclinical.com/Collateral/Documents/English-US/la....
-
- European Regulation (EC) No 726/2004. Official Journal of the European Union L 2004;136:30.4.
-
- Food and Drug Administration Amendments Act. 2007. Section 801https://clinicaltrials.gov/CT2/manage-recs/fdaaa.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
