Heparin-binding epidermal growth factor-like growth factor promotes neuroblastoma differentiation
- PMID: 28174207
- PMCID: PMC5388552
- DOI: 10.1096/fj.201600828R
Heparin-binding epidermal growth factor-like growth factor promotes neuroblastoma differentiation
Abstract
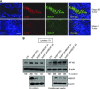
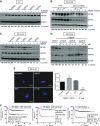
High-risk neuroblastoma is characterized by undifferentiated neuroblasts and low schwannian stroma content. The tumor stroma contributes to the suppression of tumor growth by releasing soluble factors that promote neuroblast differentiation. Here we identify heparin-binding epidermal growth factor-like growth factor (HBEGF) as a potent prodifferentiating factor in neuroblastoma. HBEGF mRNA expression is decreased in human neuroblastoma tumors compared with benign tumors, with loss correlating with decreased survival. HBEGF protein is expressed only in stromal compartments of human neuroblastoma specimens, with tissue from high-stage disease containing very little stroma or HBEGF expression. In 3 human neuroblastoma cell lines (SK-N-AS, SK-N-BE2, and SH-SY5Y), soluble HBEGF is sufficient to promote neuroblast differentiation and decrease proliferation. Heparan sulfate proteoglycans and heparin derivatives further enhance HBEGF-induced differentiation by forming a complex with the epidermal growth factor receptor, leading to activation of the ERK1/2 and STAT3 pathways and up-regulation of the inhibitor of DNA binding transcription factor. These data support a role for loss of HBEGF in the neuroblastoma tumor microenvironment in neuroblastoma pathogenesis.-Gaviglio, A. L., Knelson, E. H., Blobe, G. C. Heparin-binding epidermal growth factor-like growth factor promotes neuroblastoma differentiation.
Keywords: HBEGF; cancer; heparan sulfate; signaling; stroma.
© FASEB.
Figures




References
-
- Maris J. M., Hogarty M. D., Bagatell R., Cohn S. L. (2007) Neuroblastoma. Lancet 369, 2106–2120 - PubMed
-
- Mullassery D., Dominici C., Jesudason E. C., McDowell H. P., Losty P. D. (2009) Neuroblastoma: contemporary management. Arch. Dis. Child. Educ. Pract. Ed. 94, 177–185 - PubMed
-
- Matthay K. K., Villablanca J. G., Seeger R. C., Stram D. O., Harris R. E., Ramsay N. K., Swift P., Shimada H., Black C. T., Brodeur G. M., Gerbing R. B., Reynolds C. P.; Children’s Cancer Group (1999) Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. N. Engl. J. Med. 341, 1165–1173 - PubMed
-
- Kwiatkowski J. L., Rutkowski J. L., Yamashiro D. J., Tennekoon G. I., Brodeur G. M. (1998) Schwann cell-conditioned medium promotes neuroblastoma survival and differentiation. Cancer Res. 58, 4602–4606 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

