ATP-Dependent Persister Formation in Escherichia coli
- PMID: 28174313
- PMCID: PMC5296605
- DOI: 10.1128/mBio.02267-16
ATP-Dependent Persister Formation in Escherichia coli
Abstract
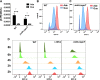
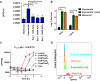
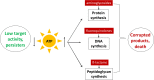
Persisters are dormant variants that form a subpopulation of cells tolerant to antibiotics. Persisters are largely responsible for the recalcitrance of chronic infections to therapy. In Escherichia coli, one widely accepted model of persister formation holds that stochastic accumulation of ppGpp causes activation of the Lon protease that degrades antitoxins; active toxins then inhibit translation, resulting in dormant, drug-tolerant persisters. We found that various stresses induce toxin-antitoxin (TA) expression but that induction of TAs does not necessarily increase persisters. The 16S rRNA promoter rrnB P1 was proposed to be a persister reporter and an indicator of toxin activation regulated by ppGpp. Using fluorescence-activated cell sorting (FACS), we confirmed the enrichment for persisters in the fraction of rrnB P1-gfp dim cells; however, this is independent of toxin-antitoxins. rrnB P1 is coregulated by ppGpp and ATP. We show that rrnB P1 can report persisters in a relA/spoT deletion background, suggesting that rrnB P1 is a persister marker responding to ATP. Consistent with this finding, decreasing the level of ATP by arsenate treatment causes drug tolerance. Lowering ATP slows translation and prevents the formation of DNA double-strand breaks upon fluoroquinolone treatment. We conclude that variation in ATP levels leads to persister formation by decreasing the activity of antibiotic targets.
Importance: Persisters are a subpopulation of antibiotic-tolerant cells responsible for the recalcitrance of chronic infections. Our current understanding of persister formation is primarily based on studies of E. coli The activation of toxin-antitoxin systems by ppGpp has become a widely accepted model for persister formation. In this study, we found that stress-induced activation of mRNA interferase-type toxins does not necessarily cause persister formation. We also found that the persister marker rrnB P1 reports persister cells because it detects a drop in cellular ATP levels. Consistent with this, lowering the ATP level decreases antibiotic target activity and, thus, leads to persister formation. We conclude that stochastic variation in ATP is the main mechanism of persister formation. A decrease in ATP provides a satisfactory explanation for the drug tolerance of persisters, since bactericidal antibiotics act by corrupting energy-dependent targets.
Copyright © 2017 Shan et al.
Figures


Comment in
-
Bacterial physiology: Persisters running out of energy.Nat Rev Microbiol. 2017 Apr;15(4):194. doi: 10.1038/nrmicro.2017.19. Epub 2017 Feb 27. Nat Rev Microbiol. 2017. PMID: 28239155 No abstract available.
Similar articles
-
Prophages and Growth Dynamics Confound Experimental Results with Antibiotic-Tolerant Persister Cells.mBio. 2017 Dec 12;8(6):e01964-17. doi: 10.1128/mBio.01964-17. mBio. 2017. PMID: 29233898 Free PMC article.
-
The Role of Integration Host Factor in Escherichia coli Persister Formation.mBio. 2022 Feb 22;13(1):e0342021. doi: 10.1128/mbio.03420-21. Epub 2022 Jan 4. mBio. 2022. PMID: 34982597 Free PMC article.
-
Specialized persister cells and the mechanism of multidrug tolerance in Escherichia coli.J Bacteriol. 2004 Dec;186(24):8172-80. doi: 10.1128/JB.186.24.8172-8180.2004. J Bacteriol. 2004. PMID: 15576765 Free PMC article.
-
Multidrug tolerance of biofilms and persister cells.Curr Top Microbiol Immunol. 2008;322:107-31. doi: 10.1007/978-3-540-75418-3_6. Curr Top Microbiol Immunol. 2008. PMID: 18453274 Review.
-
Persister cells and the riddle of biofilm survival.Biochemistry (Mosc). 2005 Feb;70(2):267-74. doi: 10.1007/s10541-005-0111-6. Biochemistry (Mosc). 2005. PMID: 15807669 Review.
Cited by
-
A Physiological Basis for Nonheritable Antibiotic Resistance.mBio. 2020 Jun 16;11(3):e00817-20. doi: 10.1128/mBio.00817-20. mBio. 2020. PMID: 32546621 Free PMC article. Review.
-
Gradients in gene essentiality reshape antibacterial research.FEMS Microbiol Rev. 2022 May 6;46(3):fuac005. doi: 10.1093/femsre/fuac005. FEMS Microbiol Rev. 2022. PMID: 35104846 Free PMC article. Review.
-
Endogenous rRNA Sequence Variation Can Regulate Stress Response Gene Expression and Phenotype.Cell Rep. 2018 Oct 2;25(1):236-248.e6. doi: 10.1016/j.celrep.2018.08.093. Cell Rep. 2018. PMID: 30282032 Free PMC article.
-
Transient Antibiotic Tolerance Triggered by Nutrient Shifts From Gluconeogenic Carbon Sources to Fatty Acid.Front Microbiol. 2022 Mar 11;13:854272. doi: 10.3389/fmicb.2022.854272. eCollection 2022. Front Microbiol. 2022. PMID: 35359720 Free PMC article.
-
Pleiotropic actions of phenothiazine drugs are detrimental to Gram-negative bacterial persister cells.Commun Biol. 2022 Mar 9;5(1):217. doi: 10.1038/s42003-022-03172-8. Commun Biol. 2022. PMID: 35264714 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

