Deletion of the Chemokine Binding Protein Gene from the Parapoxvirus Orf Virus Reduces Virulence and Pathogenesis in Sheep
- PMID: 28174562
- PMCID: PMC5258736
- DOI: 10.3389/fmicb.2017.00046
Deletion of the Chemokine Binding Protein Gene from the Parapoxvirus Orf Virus Reduces Virulence and Pathogenesis in Sheep
Abstract
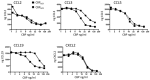
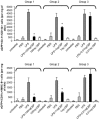
Orf virus (ORFV) is the type species of the Parapoxvirus genus of the family Poxviridae and infects sheep and goats, often around the mouth, resulting in acute pustular skin lesions. ORFV encodes several secreted immunomodulators including a broad-spectrum chemokine binding protein (CBP). Chemokines are a large family of secreted chemotactic proteins that activate and regulate inflammation induced leukocyte recruitment to sites of infection. In this study we investigated the role of CBP in vivo in the context of ORFV infection of sheep. The CBP gene was deleted from ORFV strain NZ7 and infections of sheep used to investigate the effect of CBP on pathogenesis. Animals were either infected with the wild type (wt) virus, CBP-knockout virus or revertant strains. Sheep were infected by scarification on the wool-less area of the hind legs at various doses of virus. The deletion of the CBP gene severely attenuated the virus, as only few papules formed when animals were infected with the CBP-knock-out virus at the highest dose (107 p.f.u). In contrast, large pustular lesions formed on almost all animals infected with the wt and revertant strains at 107 p.f.u. The lesions for the CBP-knock-out virus resolved approximately 5-6 days p.i, at a dose of 107 pfu whereas in animals infected with the wt and revertants at this dose, lesions began to resolve at approximately 10 days p.i. Few pustules developed at the lowest dose of 103 p.f.u. for all viruses. Immunohistochemistry of biopsy skin-tissue from pustules showed that the CBP-knockout virus replicated in all animals at the highest dose and was localized to the skin epithelium while haematoxylin and eosin staining showed histological features of the CBP-knockout virus typical of the parent virus with acanthosis, elongated rete ridges and orthokeratotic hyperkeratosis. MHC-II immunohistochemistry analysis for monocytes and dendritic cells showed greater staining within the papillary dermis of the CBP-knock-out virus compared with the revertant viruses, however this was not the case with the wt where staining was similar. Our results show that the CBP gene encodes a secreted immunodulator that has a critical role in virulence and pathogenesis.
Keywords: Orf virus; chemokine binding protein; pathogenesis; poxvirus; virulence; virus immune modulation.
Figures

Similar articles
-
Deletion of gene OV132 attenuates Orf virus more effectively than gene OV112.Appl Microbiol Biotechnol. 2023 Feb;107(2-3):835-851. doi: 10.1007/s00253-022-12323-0. Epub 2022 Dec 9. Appl Microbiol Biotechnol. 2023. PMID: 36484827 Free PMC article.
-
The chemokine-binding protein encoded by the poxvirus orf virus inhibits recruitment of dendritic cells to sites of skin inflammation and migration to peripheral lymph nodes.Cell Microbiol. 2010 May 1;12(5):665-76. doi: 10.1111/j.1462-5822.2009.01425.x. Epub 2009 Dec 21. Cell Microbiol. 2010. PMID: 20039877
-
A Broad-Spectrum Chemokine-Binding Protein of Bovine Papular Stomatitis Virus Inhibits Neutrophil and Monocyte Infiltration in Inflammatory and Wound Models of Mouse Skin.PLoS One. 2016 Dec 9;11(12):e0168007. doi: 10.1371/journal.pone.0168007. eCollection 2016. PLoS One. 2016. PMID: 27936239 Free PMC article.
-
Molecular genetic analyses of parapoxviruses pathogenic for humans.Arch Virol Suppl. 1997;13:25-34. doi: 10.1007/978-3-7091-6534-8_3. Arch Virol Suppl. 1997. PMID: 9413523 Review.
-
Secreted poxvirus chemokine binding proteins.J Leukoc Biol. 1997 Nov;62(5):570-6. doi: 10.1002/jlb.62.5.570. J Leukoc Biol. 1997. PMID: 9365110 Review.
Cited by
-
Advancing ORFV-Based Therapeutics to the Clinical Stage.Rev Med Virol. 2025 May;35(3):e70038. doi: 10.1002/rmv.70038. Rev Med Virol. 2025. PMID: 40346732 Free PMC article. Review.
-
Inactivation of indicator microorganisms and biological hazards by standard and/or alternative processing methods in Category 2 and 3 animal by-products and derived products to be used as organic fertilisers and/or soil improvers.EFSA J. 2021 Dec 2;19(12):e06932. doi: 10.2903/j.efsa.2021.6932. eCollection 2021 Dec. EFSA J. 2021. PMID: 34900004 Free PMC article.
-
Chemokine-Binding Proteins Encoded by Parapoxvirus of Red Deer of New Zealand Display Evidence of Gene Duplication and Divergence of Ligand Specificity.Front Microbiol. 2019 Jun 25;10:1421. doi: 10.3389/fmicb.2019.01421. eCollection 2019. Front Microbiol. 2019. PMID: 31293551 Free PMC article.
-
A Review on Human Orf: A Neglected Viral Zoonosis.Res Rep Trop Med. 2021 Jul 8;12:153-172. doi: 10.2147/RRTM.S306446. eCollection 2021. Res Rep Trop Med. 2021. PMID: 34267574 Free PMC article. Review.
-
The whole genomic analysis of the Orf virus strains ORFV-SC and ORFV-SC1 from the Sichuan province and their weak pathological response in rabbits.Funct Integr Genomics. 2023 May 16;23(2):163. doi: 10.1007/s10142-023-01079-z. Funct Integr Genomics. 2023. PMID: 37188892 Free PMC article.
References
-
- Achen M. G., Jeltsch M., Kukk E., Makinen T., Vitali A., Wilks A. F., et al. (1998). Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc. Natl. Acad. Sci. U.S.A. 95, 548–553. 10.1073/pnas.95.2.548 - DOI - PMC - PubMed
-
- Anderson I. E., Reid H. W., Nettleton P. F., McInnes C. J., Haig D. M. (2001). Detection of cellular cytokine mRNA expression during orf virus infection in sheep: differential interferon-gamma mRNA expression by cells in primary versus reinfection skin lesions. Vet. Immunol. Immunopathol. 83, 161–176. 10.1016/S0165-2427(01)00388-9 - DOI - PubMed
-
- Balassu T. C., Robinson A. J. (1987). Orf virus replication in bovine testis cells: kinetics of Viral DNA, polypeptide, and infectious virus production and analysis of virion polypeptides. Arch. Virol. 97, 267–281. - PubMed
-
- Bowden T. R., Coupar B. E., Babiuk S. L., White J. R., Boyd V., Duch C. J., et al. (2009). Detection of antibodies specific for sheeppox and goatpox viruses using recombinant capripoxvirus antigens in an indirect enzyme-linked immunosorbent assay. J. Virol. Methods 161, 19–29. 10.1016/j.jviromet.2009.04.031 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

