Hypoxia Positively Regulates the Expression of pH-Sensing G-Protein-Coupled Receptor OGR1 (GPR68)
- PMID: 28174749
- PMCID: PMC5247318
- DOI: 10.1016/j.jcmgh.2016.06.003
Hypoxia Positively Regulates the Expression of pH-Sensing G-Protein-Coupled Receptor OGR1 (GPR68)
Abstract
Background & aims: A novel family of proton-sensing G-protein-coupled receptors, including ovarian cancer G-protein-coupled receptor 1 (OGR1) (GPR68) has been identified to play a role in pH homeostasis. Hypoxia is known to change tissue pH as a result of anaerobic glucose metabolism through the stabilization of hypoxia-inducible factor-1α. We investigated how hypoxia regulates the expression of OGR1 in the intestinal mucosa and associated cells.
Methods: OGR1 expression in murine tumors, human colonic tissue, and myeloid cells was determined by quantitative reverse-transcription polymerase chain reaction. The influence of hypoxia on OGR1 expression was studied in monocytes/macrophages and intestinal mucosa of inflammatory bowel disease (IBD) patients. Changes in OGR1 expression in MonoMac6 (MM6) cells under hypoxia were determined upon stimulation with tumor necrosis factor (TNF), in the presence or absence of nuclear factor-κB (NF-κB) inhibitors. To study the molecular mechanisms involved, chromatin immunoprecipitation analysis of the OGR1 promoter was performed.
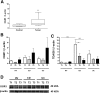
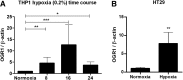
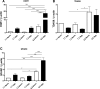
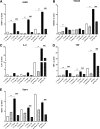
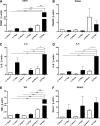
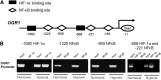
Results: OGR1 expression was significantly higher in tumor tissue compared with normal murine colon tissue. Hypoxia positively regulated the expression of OGR1 in MM6 cells, mouse peritoneal macrophages, primary human intestinal macrophages, and colonic tissue from IBD patients. In MM6 cells, hypoxia-enhanced TNF-induced OGR1 expression was reversed by inhibition of NF-κB. In addition to the effect of TNF and hypoxia, OGR1 expression was increased further at low pH. Chromatin immunoprecipitation analysis showed that HIF-1α, but not NF-κB, binds to the promoter of OGR1 under hypoxia.
Conclusions: The enhancement of TNF- and hypoxia-induced OGR1 expression under low pH points to a positive feed-forward regulation of OGR1 activity in acidic conditions, and supports a role for OGR1 in the pathogenesis of IBD.
Keywords: AICAR, 5-aminoimidazole-4-carboxamide-1-β-4-ribofuranoside; CD, Crohn's disease; ChIP, chromatin immunoprecipitation; FCS, fetal calf serum; GPR, G-protein–coupled receptor; GRP65; HIF, hypoxia-inducible factor; HV, healthy volunteer; IBD, inflammatory bowel disease; IEC, intestinal epithelial cell; IFN, interferon; IL, interleukin; Inflammation; Inflammatory Bowel Disease; MM6, MonoMac 6; NF-κB, nuclear factor-κB; OGR1, ovarian cancer G-protein–coupled receptor 1 (GPR68); Ovarian Cancer G-Protein–Coupled Receptor; RT-qPCR, quantitative reverse-transcription polymerase chain reaction; SPARC, secreted protein acidic and rich in cysteine; TDAG8; TDAG8, T-cell death-associated gene 8 (GPR65); TNF, tumor necrosis factor; Th, T-helper; UC, ulcerative colitis; WT, wild type; mRNA, messenger RNA.
Figures


References
-
- Semenza G.L. HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol. 2001;13:167–171. - PubMed
-
- Semenza G.L. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011;365:537–547. - PubMed
-
- Semenza G.L. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol. 2014;9:47–71. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

