Longitudinal Viral Dynamics in Semen During Early HIV Infection
- PMID: 28174909
- PMCID: PMC5849030
- DOI: 10.1093/cid/ciw784
Longitudinal Viral Dynamics in Semen During Early HIV Infection
Abstract
Background: Multiple viruses coinfect the male genital tract, influencing each other’s replication and perhaps affecting human immunodeficiency virus (HIV) pathogenesis and disease progression.
Methods: This study included 453 longitudinal seminal samples from 195 HIV-infected men from the San Diego Primary Infection Resource Consortium and 67 seminal samples from HIV-negative healthy controls. Seminal HIV RNA and DNA from 7 human herpesviruses (HHVs) were measured by real-time polymerase chain reaction. Longitudinal shedding rates were determined by Kaplan-Meier survival analysis. Predictors of viral shedding were determined using backwards selection in a multivariable generalized estimating equation model.
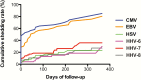
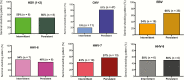
Results: HIV-infected participants presented significantly increased rates of seminal HHV shedding compared with HIV-uninfected controls. Cytomegalovirus (CMV) and Epstein-Barr virus (EBV) were the most commonly detected HHV in semen of HIV-infected participants. Persistent shedding was more common for CMV and EBV when compared to other HHVs. With exception of HHV-7, HHV shedding was not significantly influenced by HIV RNA levels, CD4+ cell counts, or antiretroviral therapy. Presence of CMV, EBV, and herpes simplex virus (HSV) were independent predictors of genital HIV RNA shedding after adjusting for plasma HIV RNA and longitudinal measurements.
Conclusions: Seminal replication of multiple HHVs is common in our HIV primary infection cohort. Genital replication of CMV and EBV was the most common and was significantly associated with seminal HIV RNA shedding. Prevalence of HSV shedding was lower and mostly intermittent, but its association with seminal HIV RNA was the strongest. Understanding the complex viral milieu in semen is important for HIV transmission but might also play a role in HIV pathogenesis and disease progression.
Figures
References
-
- Taylor S, Davies S. Antiretroviral drug concentrations in the male and female genital tract: implications for the sexual transmission of HIV. Curr Opin HIV AIDS 2010; 5:335–43. - PubMed
MeSH terms
Substances
Grants and funding
- K24 AI100665/AI/NIAID NIH HHS/United States
- R01 MH101012/MH/NIMH NIH HHS/United States
- UM1 AI069432/AI/NIAID NIH HHS/United States
- P30 AI036214/AI/NIAID NIH HHS/United States
- UL1 TR000100/TR/NCATS NIH HHS/United States
- R01 MH097520/MH/NIMH NIH HHS/United States
- U01 AI043638/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- T32 AI007384/AI/NIAID NIH HHS/United States
- DP1 DA034978/DA/NIDA NIH HHS/United States
- P30 AI027763/AI/NIAID NIH HHS/United States
- P30 MH062512/MH/NIMH NIH HHS/United States
- P01 AI074621/AI/NIAID NIH HHS/United States
- R01 MH100974/MH/NIMH NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
- R24 AI106039/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

