Piracetam Attenuates LPS-Induced Neuroinflammation and Cognitive Impairment in Rats
- PMID: 28176051
- PMCID: PMC11482115
- DOI: 10.1007/s10571-017-0468-2
Piracetam Attenuates LPS-Induced Neuroinflammation and Cognitive Impairment in Rats
Abstract
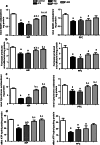
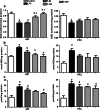
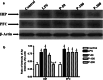
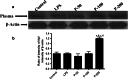
The present study was performed to investigate the effect of piracetam on neuroinflammation induced by lipopolysaccharide (LPS) and resulting changes in cognitive behavior. Neuroinflammation was induced by a single dose of LPS solution infused into each of the lateral cerebral ventricles in concentrations of 1 μg/μl, at a rate of 1 μl/min over a 5-min period, with a 5-min waiting period between the two infusions. Piracetam in doses of 50, 100, and 200 mg/kg i.p. was administered 30 min before LPS infusion and continued for 9 days. On ninth day, the behavioral test for memory and anxiety was done followed by blood collection and microdissection of the hippocampus (HIP) and prefrontal cortex brain regions. Piracetam attenuated the LPS-induced decrease in coping strategy to novel environment indicating anxiolytic activity. It also reversed the LPS-induced changes in the known arm and novel arm entries in the Y-maze test indicating amelioration of spatial memory impairment. Further, piracetam moderated LPS-induced decrease in the mitochondrial complex enzyme activities (I, II, IV, and V) and mitochondrial membrane potential. It ameliorated changes in hippocampal lipid peroxidation and nitrite levels including the activity of superoxide dismutase. Piracetam region specifically ameliorated LPS-induced increase in the level of IL-6 in HIP indicating anti-neuroinflammatory effect. Further, piracetam reduced HIP Aβ (1-40) and increased blood Aβ level suggesting efflux of Aβ from HIP to blood. Therefore, the present study indicates preclinical evidence for the use of piracetam in the treatment of neuroinflammatory disorders.
Keywords: Amyloid beta; Cognition; Lipopolysaccharide; Mitochondrial dysfunction; Neuroinflammation; Piracetam.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures




References
-
- Akdur O, Kuçuk C, Durukan P (2008) The effect of piracetam on brain damage and serum nitric oxide levels in dogs submitted to hemorrhagic shock. TJTES 14(4):277–283 - PubMed
-
- Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399. doi:10.1146/annurev.arplant.55.031903.141701 - PubMed
-
- Ben-Shaul V, Lomnitski L, Nyska A, Zurovsky Y, Bergman M, Grossman S (2001) The effect of natural antioxidants, NAO, and apocynin, on oxidative stress in the rat heart following LPS challenge. Toxicol Lett 123(1):1–10 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

