Evaluation of retinal ganglion cell function after intraocular pressure reduction measured by pattern electroretinogram in patients with primary open-angle glaucoma
- PMID: 28176172
- PMCID: PMC5364261
- DOI: 10.1007/s10633-017-9575-0
Evaluation of retinal ganglion cell function after intraocular pressure reduction measured by pattern electroretinogram in patients with primary open-angle glaucoma
Abstract
Background: To evaluate retinal ganglion cell (RGC) function after intraocular pressure (IOP) reduction measured by pattern electroretinogram (PERG) in patients with newly diagnosed, non-treated preperimetric and early stages of primary open-angle glaucoma (POAG).
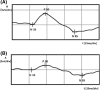
Methods: Twenty-four eyes from 24 patients with POAG: 11 eyes with preperimetric glaucoma and 13 eyes with early glaucoma received Ganfort ® (bimatoprost + timolol) once a day for a period of 1 month. Before and after the treatment, following measurements were analyzed: IOP, mean ocular perfusion pressure (MOPP), peak time of P50 and amplitude of P50 and N95 waves in PERG (ISCEV standard 2012). Correlations between PERG P50 and N95 waves, IOP and MOPP were calculated.
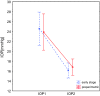
Results: After therapy, IOP significantly decreased in all eyes, on average 31%. Significant increase in MOPP in all eyes on average 14% was detected. PERG amplitude of P50 and N95 waves increased in 75 and 79% eyes, respectively, on average P50 by 28% and N95 by 38%. There were no significant interactions between the change of PERG parameters in time and stage of glaucoma.
Conclusions: Significant IOP-lowering therapy can improve RGC function measured by PERG, in patients with preperimetric and early stages of POAG.
Keywords: POAG treatment; Pattern electroretinogram; Retinal ganglion cell function.
Conflict of interest statement
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Figures


References
-
- European Glaucoma Society (2014) Terminology and guidelines for glaucoma, 4th edn
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

