Aptamer Combined with Fluorescent Silica Nanoparticles for Detection of Hepatoma Cells
- PMID: 28176286
- PMCID: PMC5296265
- DOI: 10.1186/s11671-017-1890-6
Aptamer Combined with Fluorescent Silica Nanoparticles for Detection of Hepatoma Cells
Erratum in
-
Correction to: Aptamer combined with fluorescent silica nanoparticles for detection of hepatoma cells.Discov Nano. 2025 Aug 25;20(1):146. doi: 10.1186/s11671-025-04306-7. Discov Nano. 2025. PMID: 40853420 Free PMC article. No abstract available.
Abstract
Purpose: The purpose of this study is to develop a simple, effective method to label hepatoma cells with aptamers and then detect them using fluorescent silica nanoparticles (FSNPs).
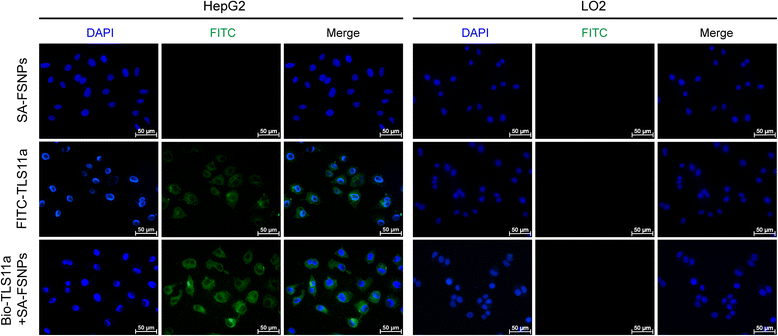
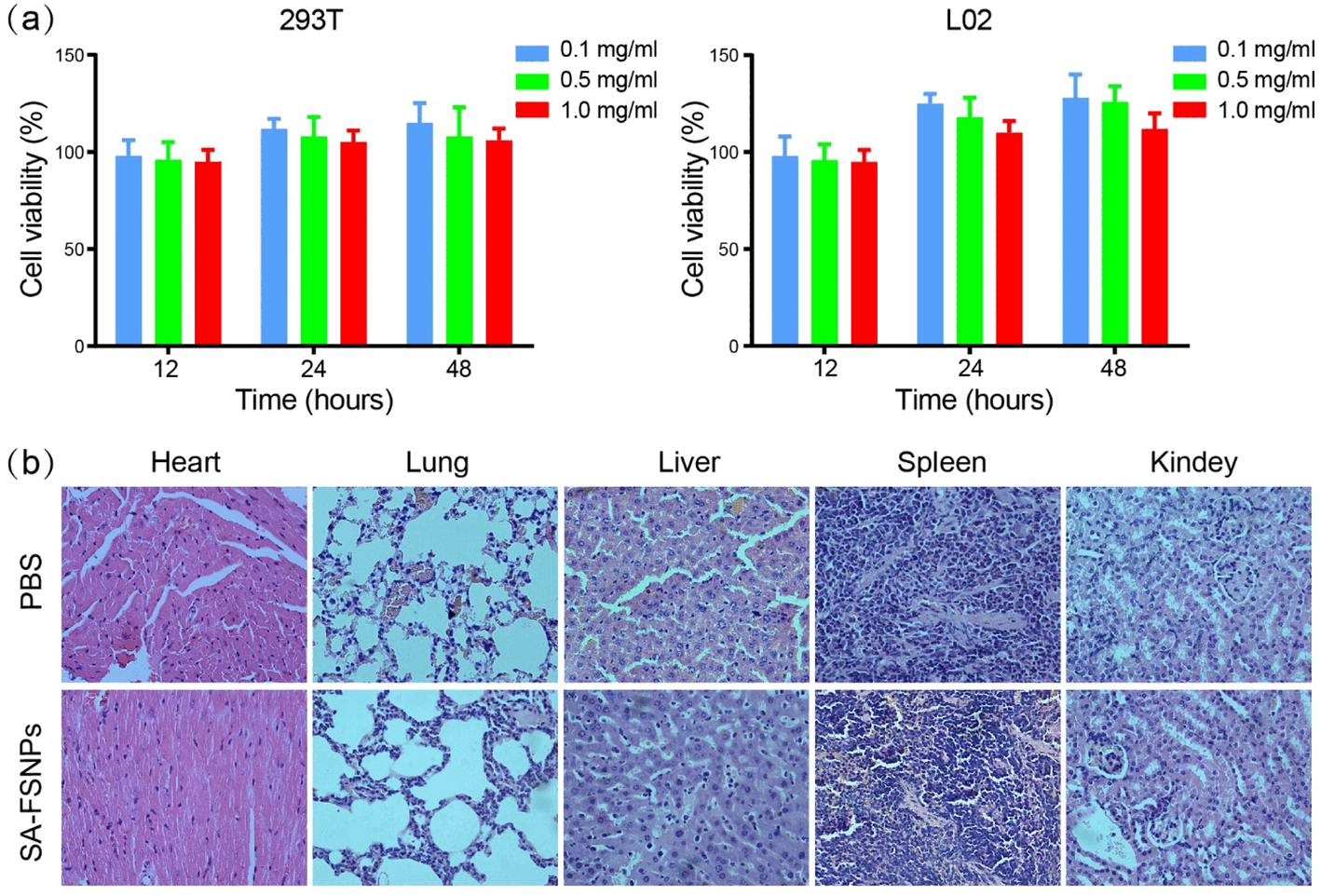
Method: Streptavidin was conjugated to carboxyl-modified fluorescein isothiocyanate (FITC)-doped silica nanoparticles which were prepared by the reverse microemulsion method. The resulting streptavidin-conjugated fluorescent silica nanoparticles (SA-FSNPs) were mixed with hepatoma cells that had been labeled with biotin-conjugated aptamer TLS11a (Bio-TLS11a). The specificity and sensitivity of the nanoprobes were assessed using flow cytometry and fluorescence microscopy. Their toxicity was assessed in normal human liver cell cultures using the MTT assay, as well as in nude mice using immunohistochemistry.
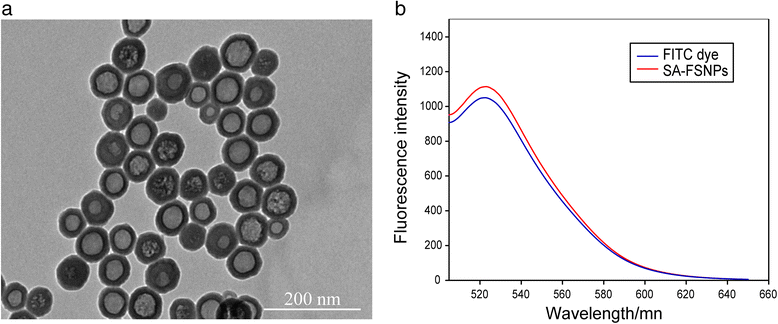
Results: SA-FSNPs showed uniform size and shape, and fluorescence properties of them was similar to the free FITC dye. SA-FSNPs were able to detect aptamer-labeled hepatoma cells with excellent specificity and good sensitivity, and they emitted strong, photobleach-resistant fluorescent signal. SA-FSNPs showed no significant toxic effects in vitro or in vivo.
Conclusion: The combination of biotin-conjugated aptamers and SA-FSNPs shows promise for sensitive detection of hepatoma cells, and potentially of other tumor cell types as well.
Keywords: Aptamer; Cancer; Fluorescent nanoparticles; Hepatoma.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Etzioni R, Urban N, Ramsey S, McIntosh M, Schwartz S, Reid B, Radich J, Anderson G, Hartwell L (2003) The case for early detection. Nat Rev Cancer 3:243–2452 - PubMed
-
- Valente K, Yacoub G, Cappellari JO, Parks G (2016) Metastatic pancreatic acinar cell carcinoma in a younger male with marked AFP production: A potential pitfall on fine needle aspiration biopsy. Diagn Cytopathol doi. doi:10.1002/dc.23610 - PubMed
-
- Sabour L, Sabour M, Ghorbian S (2016) Clinical applications of next-generation sequencing in cancer diagnosis. Pathol Oncol Res doi. doi:10.1007/s12253-016-0124-z - PubMed
-
- Ganji A, Varasteh A, Sankian M (2016) Aptamer: new arrows to target dendritic cells. J Drug Target 24:1–12 - PubMed
-
- Groff K, Brown J, Clippinger AJ (2015) Modern affinity reagents: recombinant antibodies and aptamers. Biotechnol Adv 33:1787–1798 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

