Low-dose interleukin-2 promotes STAT-5 phosphorylation, Treg survival and CTLA-4-dependent function in autoimmune liver diseases
- PMID: 28176332
- PMCID: PMC5422719
- DOI: 10.1111/cei.12940
Low-dose interleukin-2 promotes STAT-5 phosphorylation, Treg survival and CTLA-4-dependent function in autoimmune liver diseases
Abstract
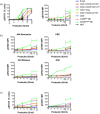
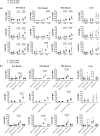
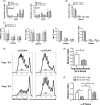
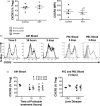
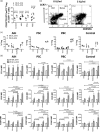
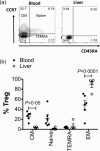
CD4+ CD25high CD127low forkhead box protein 3 (FoxP3+ ) regulatory T cells (Treg ) are essential for the maintenance of peripheral tolerance. Impaired Treg function and an imbalance between effector and Tregs contribute to the pathogenesis of autoimmune diseases. We reported recently that the hepatic microenvironment is deficient in interleukin (IL)-2, a cytokine essential for Treg survival and function. Consequently, few liver-infiltrating Treg demonstrate signal transducer and activator of transcription-5 (STAT-5) phosphorylation. To establish the potential of IL-2 to enhance Treg therapy, we investigated the effects of very low dose Proleukin (VLDP) on the phosphorylation of STAT-5 and the subsequent survival and function of Treg and T effector cells from the blood and livers of patients with autoimmune liver diseases. VLDP, at less than 5 IU/ml, resulted in selective phosphorylation of STAT-5 in Treg but not effector T cells or natural killer cells and associated with increased expression of cytotoxic T lymphocyte antigen-4 (CTLA-4), FoxP3 and CD25 and the anti-apoptotic protein Bcl-2 in Treg with the greatest enhancement of regulatory phenotype in the effector memory Treg population. VLDP also maintained expression of the liver-homing chemokine receptor CXCR3. VLDP enhanced Treg function in a CTLA-4-dependent manner. These findings open new avenues for future VLDP cytokine therapy alone or in combination with clinical grade Treg in autoimmune liver diseases, as VLDP could not only enhance regulatory phenotype and functional property but also the survival of intrahepatic Treg .
Keywords: Bcl-2; CTLA-4; STAT-5; autoimmune liver disease; interleukin-2; regulatory T cells.
© 2017 The Authors. Clinical & Experimental Immunology published by John Wiley & Sons Ltd on behalf of British Society for Immunology.
Figures

Similar articles
-
Sustained in vivo signaling by long-lived IL-2 induces prolonged increases of regulatory T cells.J Autoimmun. 2015 Jan;56:66-80. doi: 10.1016/j.jaut.2014.10.002. Epub 2014 Oct 30. J Autoimmun. 2015. PMID: 25457307 Free PMC article.
-
A long-lived IL-2 mutein that selectively activates and expands regulatory T cells as a therapy for autoimmune disease.J Autoimmun. 2018 Dec;95:1-14. doi: 10.1016/j.jaut.2018.10.017. Epub 2018 Nov 13. J Autoimmun. 2018. PMID: 30446251 Free PMC article.
-
Comparative dose-responses of recombinant human IL-2 and IL-7 on STAT5 phosphorylation in CD4+FOXP3- cells versus regulatory T cells: a whole blood perspective.Cytokine. 2014 Sep;69(1):146-9. doi: 10.1016/j.cyto.2014.05.021. Epub 2014 Jun 16. Cytokine. 2014. PMID: 24947990
-
The role of FOXP3+ regulatory T cells in human autoimmune and inflammatory diseases.Clin Exp Immunol. 2019 Jul;197(1):24-35. doi: 10.1111/cei.13288. Epub 2019 Mar 24. Clin Exp Immunol. 2019. PMID: 30830965 Free PMC article. Review.
-
Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease.Immunol Rev. 2006 Aug;212:8-27. doi: 10.1111/j.0105-2896.2006.00427.x. Immunol Rev. 2006. PMID: 16903903 Review.
Cited by
-
Metabolic Optimisation of Regulatory T Cells in Transplantation.Front Immunol. 2020 Sep 2;11:2005. doi: 10.3389/fimmu.2020.02005. eCollection 2020. Front Immunol. 2020. PMID: 33013855 Free PMC article. Review.
-
Similarities and divergences in the metabolism of immune cells in cancer and helminthic infections.Front Oncol. 2023 Nov 16;13:1251355. doi: 10.3389/fonc.2023.1251355. eCollection 2023. Front Oncol. 2023. PMID: 38044996 Free PMC article. Review.
-
Immunopathobiology and therapeutic targets related to cytokines in liver diseases.Cell Mol Immunol. 2021 Jan;18(1):18-37. doi: 10.1038/s41423-020-00580-w. Epub 2020 Nov 17. Cell Mol Immunol. 2021. PMID: 33203939 Free PMC article. Review.
-
The dual role of TIGIT in regulatory and effector T cells in chronic liver disease.JHEP Rep. 2025 Mar 29;7(6):101405. doi: 10.1016/j.jhepr.2025.101405. eCollection 2025 Jun. JHEP Rep. 2025. PMID: 40496440 Free PMC article.
-
Dysfunctional Immune Regulation in Autoimmune Hepatitis: From Pathogenesis to Novel Therapies.Front Immunol. 2021 Sep 28;12:746436. doi: 10.3389/fimmu.2021.746436. eCollection 2021. Front Immunol. 2021. PMID: 34650567 Free PMC article. Review.
References
-
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self‐tolerance maintained by activated T cells expressing IL‐2 receptor alpha‐chains (CD25). Breakdown of a single mechanism of self‐tolerance causes various autoimmune diseases. J Immunol 1995; 155:1151–64. - PubMed
-
- Sebode M, Peiseler M, Franke B et al Reduced FOXP3(+) regulatory T cells in patients with primary sclerosing cholangitis are associated with IL2RA gene polymorphisms. J Hepatol 2014; 60:1010–6. - PubMed
-
- Lan RY, Cheng C, Lian ZX et al Liver‐targeted and peripheral blood alterations of regulatory T cells in primary biliary cirrhosis. Hepatology 2006; 43:729–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

