Unusual life cycle and impact on microfibril assembly of ADAMTS17, a secreted metalloprotease mutated in genetic eye disease
- PMID: 28176809
- PMCID: PMC5296908
- DOI: 10.1038/srep41871
Unusual life cycle and impact on microfibril assembly of ADAMTS17, a secreted metalloprotease mutated in genetic eye disease
Abstract
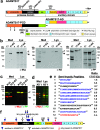
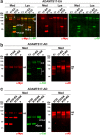
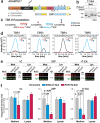
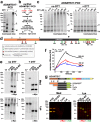
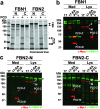
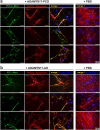
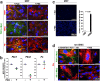
Secreted metalloproteases have diverse roles in the formation, remodeling, and the destruction of extracellular matrix. Recessive mutations in the secreted metalloprotease ADAMTS17 cause ectopia lentis and short stature in humans with Weill-Marchesani-like syndrome and primary open angle glaucoma and ectopia lentis in dogs. Little is known about this protease or its connection to fibrillin microfibrils, whose major component, fibrillin-1, is genetically associated with ectopia lentis and alterations in height. Fibrillin microfibrils form the ocular zonule and are present in the drainage apparatus of the eye. We show that recombinant ADAMTS17 has unique characteristics and an unusual life cycle. It undergoes rapid autocatalytic processing in trans after its secretion from cells. Secretion of ADAMTS17 requires O-fucosylation and its autocatalytic activity does not depend on propeptide processing by furin. ADAMTS17 binds recombinant fibrillin-2 but not fibrillin-1 and does not cleave either. It colocalizes to fibrillin-1 containing microfibrils in cultured fibroblasts and suppresses fibrillin-2 (FBN2) incorporation in microfibrils, in part by transcriptional downregulation of Fbn2 mRNA expression. RNA in situ hybridization detected Adamts17 expression in specific structures in the eye, skeleton and other organs, where it may regulate the fibrillin isoform composition of microfibrils.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Bekhouche M. & Colige A. The procollagen N-proteinases ADAMTS2, 3 and 14 in pathophysiology. Matrix Biol. 44–46, 46–53 (2015). - PubMed
-
- Fujikawa K., Suzuki H., McMullen B. & Chung D. Purification of human von Willebrand factor-cleaving protease and its identification as a new member of the metalloproteinase family. Blood 98, 1662–1666. (2001). - PubMed
-
- Stanton H. et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature 434, 648–652 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

