Structure of an octameric form of the minichromosome maintenance protein from the archaeon Pyrococcus abyssi
- PMID: 28176822
- PMCID: PMC5296750
- DOI: 10.1038/srep42019
Structure of an octameric form of the minichromosome maintenance protein from the archaeon Pyrococcus abyssi
Abstract
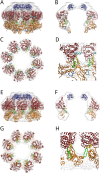
Cell division is a complex process that requires precise duplication of genetic material. Duplication is concerted by replisomes. The Minichromosome Maintenance (MCM) replicative helicase is a crucial component of replisomes. Eukaryotic and archaeal MCM proteins are highly conserved. In fact, archaeal MCMs are powerful tools for elucidating essential features of MCM function. However, while eukaryotic MCM2-7 is a heterocomplex made of different polypeptide chains, the MCM complexes of many Archaea form homohexamers from a single gene product. Moreover, some archaeal MCMs are polymorphic, and both hexameric and heptameric architectures have been reported for the same polypeptide. Here, we present the structure of the archaeal MCM helicase from Pyrococcus abyssi in its single octameric ring assembly. To our knowledge, this is the first report of a full-length octameric MCM helicase.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

