Emerging roles of ER stress and unfolded protein response pathways in skeletal muscle health and disease
- PMID: 28177127
- PMCID: PMC5548649
- DOI: 10.1002/jcp.25852
Emerging roles of ER stress and unfolded protein response pathways in skeletal muscle health and disease
Abstract
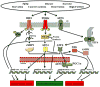
Skeletal muscle is the most abundant tissue in the human body and can adapt its mass as a consequence of physical activity, metabolism, growth factors, and disease conditions. Skeletal muscle contains an extensive network of endoplasmic reticulum (ER), called sarcoplasmic reticulum, which plays an important role in the regulation of proteostasis and calcium homeostasis. In many cell types, environmental and genetic factors that disrupt ER function cause an accumulation of misfolded and unfolded proteins in the ER lumen that ultimately leads to ER stress. To alleviate the stress and restore homeostasis, the ER activates a signaling network called the unfolded protein response (UPR). The UPR has three arms, which regulate protein synthesis and expression of many ER chaperone and regulatory proteins. However, the role of individual UPR pathways in skeletal muscle has just begun to be investigated. Recent studies suggest that UPR pathways play pivotal roles in muscle stem cell homeostasis, myogenic differentiation, and regeneration of injured skeletal muscle. Moreover, markers of ER stress and the UPR are activated in skeletal muscle in diverse conditions such as exercise, denervation, starvation, high fat diet, cancer cachexia, and aging. Accumulating evidence also suggests that ER stress may have important roles in the pathogenesis of inflammatory myopathies and genetic muscle disorders. The purpose of this review article is to discuss the role and potential mechanisms by which ER stress and the individual arms of the UPR regulate skeletal muscle formation, plasticity, and function in various physiological and pathophysiological conditions.
Keywords: ER stress; UPR; atrophy; myogenesis; skeletal muscle.
© 2017 Wiley Periodicals, Inc.
Figures

References
-
- Acosta-Alvear D, Zhou Y, Blais A, Tsikitis M, Lents NH, Arias C, Lennon CJ, Kluger Y, Dynlacht BD. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol Cell. 2007;27(1):53–66. - PubMed
-
- Askanas V, Engel WK. Inclusion-body myositis: newest concepts of pathogenesis and relation to aging and Alzheimer disease. J Neuropathol Exp Neurol. 2001;60(1):1–14. - PubMed
-
- Askanas V, Engel WK. Inclusion-body myositis and myopathies: different etiologies, possibly similar pathogenic mechanisms. Curr Opin Neurol. 2002;15(5):525–531. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

