A Chaperone Complex Formed by HSP47, FKBP65, and BiP Modulates Telopeptide Lysyl Hydroxylation of Type I Procollagen
- PMID: 28177155
- PMCID: PMC5466459
- DOI: 10.1002/jbmr.3095
A Chaperone Complex Formed by HSP47, FKBP65, and BiP Modulates Telopeptide Lysyl Hydroxylation of Type I Procollagen
Abstract
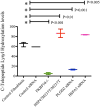
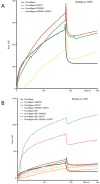
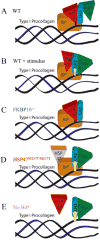
Lysine hydroxylation of type I collagen telopeptides varies from tissue to tissue, and these distinct hydroxylation patterns modulate collagen cross-linking to generate a unique extracellular matrix. Abnormalities in these patterns contribute to pathologies that include osteogenesis imperfecta (OI), fibrosis, and cancer. Telopeptide procollagen modifications are carried out by lysyl hydroxylase 2 (LH2); however, little is known regarding how this enzyme regulates hydroxylation patterns. We identified an ER complex of resident chaperones that includes HSP47, FKBP65, and BiP regulating the activity of LH2. Our findings show that FKBP65 and HSP47 modulate the activity of LH2 to either favor or repress its activity. BiP was also identified as a member of the complex, playing a role in enhancing the formation of the complex. This newly identified ER chaperone complex contributes to our understanding of how LH2 regulates lysyl hydroxylation of type I collagen C-telopeptides to affect the quality of connective tissues. © 2017 American Society for Bone and Mineral Research.
© 2017 American Society for Bone and Mineral Research.
Conflict of interest statement
Figures



References
-
- Walker LC, Overstreet MA, Yeowell HN. Tissue-specific expression and regulation of the alternatively-spliced forms of lysyl hydroxylase 2 (LH2) in human kidney cells and skin fibroblasts. Matrix Biol. 2005 Jan;23(8):515–523. - PubMed
-
- van der Slot AJ, Zuurmond AM, van den Bogaerdt AJ, Ulrich MMW, Middelkoop E, Boers W, et al. Increased formation of pyridinoline cross-links due to higher telopeptide lysyl hydroxylase levels is a general fibrotic phenomenon. Matrix Biol. 2004 Jul;23(4):251–257. - PubMed
-
- Remst DFG, Blaney Davidson EN, Vitters EL, Blom AB, Stoop R, Snabel JM, et al. Osteoarthritis-related fibrosis is associated with both elevated pyridinoline cross-link formation and lysyl hydroxylase 2b expression. Osteoarthr Cartil. 2013 Jan;21(1):157–164. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

