Glycoblocks: a schematic three-dimensional representation for glycans and their interactions
- PMID: 28177314
- PMCID: PMC5297921
- DOI: 10.1107/S2059798316013553
Glycoblocks: a schematic three-dimensional representation for glycans and their interactions
Abstract
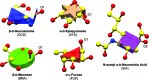
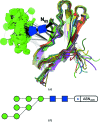
The close-range interactions provided by covalently linked glycans are essential for the correct folding of glycoproteins and also play a pivotal role in recognition processes. Being able to visualise protein-glycan and glycan-glycan contacts in a clear way is thus of great importance for the understanding of these biological processes. In structural terms, glycosylation sugars glue the protein together via hydrogen bonds, whereas non-covalently bound glycans frequently harness additional stacking interactions. Finding an unobscured molecular view of these multipartite scenarios is usually far from trivial; in addition to the need to show the interacting protein residues, glycans may contain many branched sugars, each composed of more than ten non-H atoms and offering more than three potential bonding partners. With structural glycoscience finally gaining popularity and steadily increasing the deposition rate of three-dimensional structures of glycoproteins, the need for a clear way of depicting these interactions is more pressing than ever. Here a schematic representation, named Glycoblocks, is introduced which combines a simplified bonding-network depiction (covering hydrogen bonds and stacking interactions) with the familiar two-dimensional glycan notation used by the glycobiology community, brought into three dimensions by the CCP4 molecular graphics project (CCP4mg).
Keywords: CCP4mg; Glycoblocks; Privateer; carbohydrates; glycans; interactions; molecular graphics; three-dimensional representations.
Figures






Similar articles
-
Analysis and validation of overall N-glycan conformation in Privateer.Acta Crystallogr D Struct Biol. 2023 Jun 1;79(Pt 6):462-472. doi: 10.1107/S2059798323003510. Epub 2023 May 23. Acta Crystallogr D Struct Biol. 2023. PMID: 37219590 Free PMC article.
-
Quantitative O-glycomics based on improvement of the one-pot method for nonreductive O-glycan release and simultaneous stable isotope labeling with 1-(d0/d5)phenyl-3-methyl-5-pyrazolone followed by mass spectrometric analysis.J Proteomics. 2017 Jan 6;150:18-30. doi: 10.1016/j.jprot.2016.08.012. Epub 2016 Aug 29. J Proteomics. 2017. PMID: 27585995
-
Function and 3D structure of the N-glycans on glycoproteins.Int J Mol Sci. 2012;13(7):8398-8429. doi: 10.3390/ijms13078398. Epub 2012 Jul 6. Int J Mol Sci. 2012. PMID: 22942711 Free PMC article. Review.
-
Chemical reporters to study mammalian O-glycosylation.Biochem Soc Trans. 2021 Apr 30;49(2):903-913. doi: 10.1042/BST20200839. Biochem Soc Trans. 2021. PMID: 33860782 Free PMC article. Review.
-
The clinical role of glycobiology on ovarian cancer progression.Adv Cancer Res. 2023;157:1-22. doi: 10.1016/bs.acr.2022.07.004. Epub 2022 Aug 18. Adv Cancer Res. 2023. PMID: 36725106 Review.
Cited by
-
Novel Inhibitory Function of the Rhizomucor miehei Lipase Propeptide and Three-Dimensional Structures of Its Complexes with the Enzyme.ACS Omega. 2019 Jun 7;4(6):9964-9975. doi: 10.1021/acsomega.9b00612. eCollection 2019 Jun 30. ACS Omega. 2019. PMID: 31460089 Free PMC article.
-
Dynamic and Functional Profiling of Xylan-Degrading Enzymes in Aspergillus Secretomes Using Activity-Based Probes.ACS Cent Sci. 2019 Jun 26;5(6):1067-1078. doi: 10.1021/acscentsci.9b00221. Epub 2019 May 24. ACS Cent Sci. 2019. PMID: 31263766 Free PMC article.
-
Strategies for carbohydrate model building, refinement and validation.Acta Crystallogr D Struct Biol. 2017 Feb 1;73(Pt 2):171-186. doi: 10.1107/S2059798316016910. Epub 2017 Feb 1. Acta Crystallogr D Struct Biol. 2017. PMID: 28177313 Free PMC article.
-
A hierarchical structure in the N-glycosylation process governs the N-glycosylation output: prolonged cultivation induces glycoenzymes expression variations that are reflected in the cellular N-glycome but not in the protein and site-specific glycoprofile of CHO cells.Glycobiology. 2024 Jun 22;34(8):cwae045. doi: 10.1093/glycob/cwae045. Glycobiology. 2024. PMID: 38938083 Free PMC article.
-
Activity of botulinum neurotoxin X and its structure when shielded by a non-toxic non-hemagglutinin protein.Commun Chem. 2024 Aug 13;7(1):179. doi: 10.1038/s42004-024-01262-8. Commun Chem. 2024. PMID: 39138288 Free PMC article.
References
-
- Agirre, J., Davies, G., Wilson, K. & Cowtan, K. (2015). Nat. Chem. Biol. 11, 303. - PubMed
-
- Agirre, J., Iglesias-Fernández, J., Rovira, C., Davies, G. J., Wilson, K. S. & Cowtan, K. D. (2015). Nat. Struct. Mol. Biol. 22, 833–834. - PubMed
-
- Ardá, A., Blasco, P., Varón Silva, D., Schubert, V., André, S., Bruix, M., Cañada, F. J., Gabius, H. J., Unverzagt, C. & Jiménez-Barbero, J. (2013). J. Am. Chem. Soc. 135, 2667–2675. - PubMed
-
- Berman, H., Henrick, K. & Nakamura, H. (2003). Nat. Struct. Biol. 10, 980. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

