Cyclin D mediates tolerance of genome-doubling in cancers with functional p53
- PMID: 28177473
- PMCID: PMC5391719
- DOI: 10.1093/annonc/mdw612
Cyclin D mediates tolerance of genome-doubling in cancers with functional p53
Abstract
Background: Aneuploidy and chromosomal instability (CIN) are common features of human malignancy that fuel genetic heterogeneity. Although tolerance to tetraploidization, an intermediate state that further exacerbates CIN, is frequently mediated by TP53 dysfunction, we find that some genome-doubled tumours retain wild-type TP53. We sought to understand how tetraploid cells with a functional p53/p21-axis tolerate genome-doubling events.
Methods: We performed quantitative proteomics in a diploid/tetraploid pair within a system of multiple independently derived TP53 wild-type tetraploid clones arising spontaneously from a diploid progenitor. We characterized adapted and acute tetraploidization in a variety of flow cytometry and biochemical assays and tested our findings against human tumours through bioinformatics analysis of the TCGA dataset.
Results: Cyclin D1 was found to be specifically overexpressed in early but not late passage tetraploid clones, and this overexpression was sufficient to promote tolerance to spontaneous and pharmacologically induced tetraploidy. We provide evidence that this role extends to D-type cyclins and their overexpression confers specific proliferative advantage to tetraploid cells. We demonstrate that tetraploid clones exhibit elevated levels of functional p53 and p21 but override the p53/p21 checkpoint by elevated expression of cyclin D1, via a stoichiometry-dependent and CDK activity-independent mechanism. Tetraploid cells do not exhibit increased sensitivity to abemaciclib, suggesting that cyclin D-overexpressing tumours might not be specifically amenable to treatment with CDK4/6 inhibitors.
Conclusions: Our study suggests that D-type cyclin overexpression is an acute event, permissive for rapid adaptation to a genome-doubled state in TP53 wild-type tumours and that its overexpression is dispensable in later stages of tumour progression.
Figures
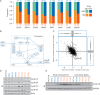
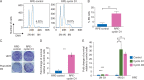
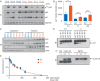

References
-
- Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. et al. Clonogenic assay of cells in vitro. Nature protocols 2006; 1(5): 2315–2319. Epub 2007/04/05. doi: 10.1038/nprot.2006.339. PubMed PMID: 17406473. - PubMed
-
- Ganem NJ, Storchova Z, Pellman D. Tetraploidy, aneuploidy and cancer. Curr Opin Genet Dev 2007; 17(2): 157–162. - PubMed
-
- Di Leonardo A, Khan SH, Linke SP. et al. Rereplication in the presence of mitotic spindle inhibitors in human and mouse fibroblasts lacking either p53 or pRb function. Cancer Res 1997; 57(6): 1013–1019. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

