Rapid, sensitive detection of bacteria in platelet samples with Fountain Flow Cytometry
- PMID: 28177537
- PMCID: PMC6817245
- DOI: 10.1002/jcla.22115
Rapid, sensitive detection of bacteria in platelet samples with Fountain Flow Cytometry
Abstract
Background: There is a current need to develop a technique for bacterial screening of platelet donations that is more rapid, sensitive, and economical than alternatives. The objective of this research was to perform a pilot test of the viability of Fountain Flow Cytometry (FFC), for the rapid and sensitive detection of bacteria in platelet donations.
Methods: Platelet samples were inoculated with serial dilutions of five selected bacterial strains. Samples were then centrifuged, reconstituted in buffer, and stained with a live/dead bacterial stain cocktail. The resulting aqueous sample was measured by FFC, in which the sample passed as a stream in front of an LED, which excited the fluorescent labels. Fluorescence was detected with a digital camera as the sample flowed toward it.
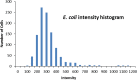
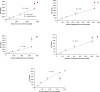
Results: Fountain Flow Cytometry enumeration yielded results that were linear with bacterial concentration, having an R2 of ≥0.98 with a detection efficiency of 92%±3%. Measurements of uninoculated samples showed a false-positive detection rate at ~400 colony forming units (CFU)/mL. Detection of bacterial concentrations in platelets above this threshold can be made in ~15 minutes, including sample preparation time.
Conclusion: This pilot study supports the efficacy of FFC for the rapid and sensitive screening of platelet donations for bacteria.
Keywords: bacteria; biodetection; cytometry; fountain flow; platelets.
© 2017 Wiley Periodicals, Inc.
Figures

References
-
- Whitaker BI, Hinkins S. The 2011 national blood collection and utilization survey report (Internet). US Department of Health and Human Services. 2011. Available from: http://www.hhs.gov/ash/bloodsafety/2011-nbcus.pdf. Accessed January 7, 2014.
-
- CDC . Fatal bacterial infections associated with platelet transfusion‐United States. Morb Mortal Wkly Rep 2005;54:168–170. - PubMed
-
- Brecher ME, Jacobs MR, Katz LM, et al. AABB Bacterial Contamination Task Force. Survey of methods used to detect bacterial contamination of platelet products in the United States in 2011. Transfusion. 2013;53:911–918. - PubMed
-
- Dreier J, Vollmer T, Kleesiek K. Novel flow cytometry–based screening for bacterial contamination of donor platelet preparations compared with other rapid screening methods. Clin Chem. 2009;55:1492–1502. - PubMed
-
- Heaton WA, Good CE, Galloway‐Haskins R, et al. Evaluation of a rapid colorimetric assay for detection of bacterial contamination in apheresis and pooled random‐donor platelet units. Transfusion. 2014;54:1634–1641. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

