The oxidized thiol proteome in aging and cataractous mouse and human lens revealed by ICAT labeling
- PMID: 28177569
- PMCID: PMC5334568
- DOI: 10.1111/acel.12548
The oxidized thiol proteome in aging and cataractous mouse and human lens revealed by ICAT labeling
Abstract
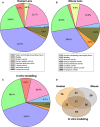
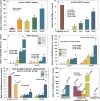
Age-related cataractogenesis is associated with disulfide-linked high molecular weight (HMW) crystallin aggregates. We recently found that the lens crystallin disulfidome was evolutionarily conserved in human and glutathione-depleted mouse (LEGSKO) cataracts and that it could be mimicked by oxidation in vitro (Mol. Cell Proteomics, 14, 3211-23 (2015)). To obtain a comprehensive blueprint of the oxidized key regulatory and cytoskeletal proteins underlying cataractogenesis, we have now used the same approach to determine, in the same specimens, all the disulfide-forming noncrystallin proteins identified by ICAT proteomics. Seventy-four, 50, and 54 disulfide-forming proteins were identified in the human and mouse cataracts and the in vitro oxidation model, respectively, of which 17 were common to all three groups. Enzymes with oxidized cysteine at critical sites include GAPDH (hGAPDH, Cys247), glutathione synthase (hGSS, Cys294), aldehyde dehydrogenase (hALDH1A1, Cys126 and Cys186), sorbitol dehydrogenase (hSORD, Cys140, Cys165, and Cys179), and PARK7 (hPARK7, Cys46 and Cys53). Extensive oxidation was also present in lens-specific intermediate filament proteins, such as BFSP1 and BFSP12 (hBFSP1 and hBFSP12, Cys167, Cys65, and Cys326), vimentin (mVim, Cys328), and cytokeratins, as well as microfilament and microtubule filament proteins, such as tubulin and actins. While the biological impact of these modifications for lens physiology remains to be determined, many of these oxidation sites have already been associated with either impaired metabolism or cytoskeletal architecture, strongly suggesting that they have a pathogenic role in cataractogenesis. By extrapolation, these findings may be of broader significance for age- and disease-related dysfunctions associated with oxidant stress.
Keywords: aging; cataractogenesis; disulfide; mass spectrometry; proteomics; reactive oxygen species.
© 2016 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures




Similar articles
-
Evidence of Highly Conserved β-Crystallin Disulfidome that Can be Mimicked by In Vitro Oxidation in Age-related Human Cataract and Glutathione Depleted Mouse Lens.Mol Cell Proteomics. 2015 Dec;14(12):3211-23. doi: 10.1074/mcp.M115.050948. Epub 2015 Oct 9. Mol Cell Proteomics. 2015. PMID: 26453637 Free PMC article.
-
Reactive cysteine residues in the oxidative dimerization and Cu2+ induced aggregation of human γD-crystallin: Implications for age-related cataract.Biochim Biophys Acta Mol Basis Dis. 2018 Nov;1864(11):3595-3604. doi: 10.1016/j.bbadis.2018.08.021. Epub 2018 Aug 18. Biochim Biophys Acta Mol Basis Dis. 2018. PMID: 30251679 Free PMC article.
-
Stepwise oxidations play key roles in the structural and functional regulations of DJ-1.Biochem J. 2021 Oct 15;478(19):3505-3525. doi: 10.1042/BCJ20210245. Biochem J. 2021. PMID: 34515295 Free PMC article.
-
Protein posttranslational modification (PTM) by glycation: Role in lens aging and age-related cataractogenesis.Exp Eye Res. 2021 Sep;210:108705. doi: 10.1016/j.exer.2021.108705. Epub 2021 Jul 20. Exp Eye Res. 2021. PMID: 34297945 Free PMC article. Review.
-
Thiol regulation in the lens.J Ocul Pharmacol Ther. 2000 Apr;16(2):137-48. doi: 10.1089/jop.2000.16.137. J Ocul Pharmacol Ther. 2000. PMID: 10803424 Review.
Cited by
-
The Possible Positive Mechanisms of Pirenoxine in Cataract Formation.Int J Mol Sci. 2022 Aug 21;23(16):9431. doi: 10.3390/ijms23169431. Int J Mol Sci. 2022. PMID: 36012695 Free PMC article. Review.
-
Oxidative Stress in Cataract Formation: Is There a Treatment Approach on the Horizon?Antioxidants (Basel). 2024 Oct 16;13(10):1249. doi: 10.3390/antiox13101249. Antioxidants (Basel). 2024. PMID: 39456502 Free PMC article. Review.
-
Halogenation of tyrosine perturbs large-scale protein self-organization.Nat Commun. 2022 Aug 17;13(1):4843. doi: 10.1038/s41467-022-32535-2. Nat Commun. 2022. PMID: 35977922 Free PMC article.
-
M6A-modified BFSP1 induces aerobic glycolysis to promote liver cancer growth and metastasis through upregulating tropomodulin 4.Mol Biomed. 2025 Mar 18;6(1):17. doi: 10.1186/s43556-025-00256-9. Mol Biomed. 2025. PMID: 40097750 Free PMC article.
-
Lens Cytoskeleton: An Update on the Etiopathogenesis of Human Cataracts.Cureus. 2024 Mar 23;16(3):e56793. doi: 10.7759/cureus.56793. eCollection 2024 Mar. Cureus. 2024. PMID: 38650819 Free PMC article.
References
-
- Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414, 813–820. - PubMed
-
- Conley YP, Erturk D, Keverline A, Mah TS, Keravala A, Barnes LR, Bruchis A, Hess JF, FitzGerald PG, Weeks DE, Ferrell RE, Gorin MB (2000) A juvenile‐onset, progressive cataract locus on chromosome 3q21‐q22 is associated with a missense mutation in the beaded filament structural protein‐2. Am. J. Hum. Genet. 66, 1426–1431. - PMC - PubMed
-
- Cumming RC, Schubert D (2005) Amyloid‐beta induces disulfide bonding and aggregation of GAPDH in Alzheimer's disease. FASEB J. 19, 2060–2062. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

