Photoinduced Reductive Elimination of H2 from the Nitrogenase Dihydride (Janus) State Involves a FeMo-cofactor-H2 Intermediate
- PMID: 28177622
- PMCID: PMC5444871
- DOI: 10.1021/acs.inorgchem.6b02899
Photoinduced Reductive Elimination of H2 from the Nitrogenase Dihydride (Janus) State Involves a FeMo-cofactor-H2 Intermediate
Abstract
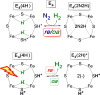
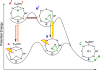
N2 reduction by nitrogenase involves the accumulation of four reducing equivalents at the active site FeMo-cofactor to form a state with two [Fe-H-Fe] bridging hydrides (denoted E4(4H), the Janus intermediate), and we recently demonstrated that the enzyme is activated to cleave the N≡N triple bond by the reductive elimination (re) of H2 from this state. We are exploring a photochemical approach to obtaining atomic-level details of the re activation process. We have shown that, when E4(4H) at cryogenic temperatures is subjected to 450 nm irradiation in an EPR cavity, it cleanly undergoes photoinduced re of H2 to give a reactive doubly reduced intermediate, denoted E4(2H)*, which corresponds to the intermediate that would form if thermal dissociative re loss of H2 preceded N2 binding. Experiments reported here establish that photoinduced re primarily occurs in two steps. Photolysis of E4(4H) generates an intermediate state that undergoes subsequent photoinduced conversion to [E4(2H)* + H2]. The experiments, supported by DFT calculations, indicate that the trapped intermediate is an H2 complex on the ground adiabatic potential energy suface that connects E4(4H) with [E4(2H)* + H2]. We suggest that this complex, denoted E4(H2; 2H), is a thermally populated intermediate in the catalytically central re of H2 by E4(4H) and that N2 reacts with this complex to complete the activated conversion of [E4(4H) + N2] into [E4(2N2H) + H2].
Figures









References
-
-
A knowledgeable reviewer noted that a more common notation uses capitals in the abbreviations (RE, OA), but we feel that it is beneficial to retain consistency with our earlier reports.
-
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

