Comparison between transrectal and transperineal prostate biopsy for detection of prostate cancer: a meta-analysis and trial sequential analysis
- PMID: 28177897
- PMCID: PMC5410307
- DOI: 10.18632/oncotarget.15056
Comparison between transrectal and transperineal prostate biopsy for detection of prostate cancer: a meta-analysis and trial sequential analysis
Abstract
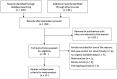
To systematically assess the efficacy and complications of transrectal (TR) versus transperineal (TP) prostate biopsy in the detection of prostate cancer (PCa). A meta-analysis was performed by searching the databases Pubmed, Embase and Web of science for the relevant available studies until September 1st, 2016, and thirteen studies met the inclusion criteria. The pooled odds ratios with 95% confidence intervals were calculated to evaluate the differences of TR and TP groups in PCa detection rate. Then, trial sequential analysis was performed to reduce the risk of type I error and estimated whether the evidence of the results was reliable. Overall, this meta-analysis included a total of 4280 patients, who had been accrued between April 2000 and Aug 2014 and randomly divided into TR group and TP group. Prostate biopsies included sextant, extensive and saturation biopsy procedures. Patients who received TP prostate biopsy had no significant improvement in PCa detection rate, comparing TR group. Moreover, when comparing TR and TP studies, no significant difference was found in abnormal DRE findings, serum PSA level measurement, Gleason score, prostate volume. Besides, this meta-analysis showed no obvious differences between these two groups in terms of relevant complications. Therefore, this meta-analysis revealed that no significant differences were found in PCa detection rate between TP and TR approaches for prostate biopsy. However, with regard to pain relief and additional anesthesia, TR prostate needle biopsy was relatively preferable, compared to TP prostate biopsy.
Keywords: meta-analysis; prostate biopsy; prostate cancer; transperineal; transrectal.
Conflict of interest statement
We declare that we have no conflict of interest.
Figures








References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. - PubMed
-
- Eeles RA, Olama AA, Benlloch S, Saunders EJ, Leongamornlert DA, Tymrakiewicz M, Ghoussaini M, Luccarini C, Dennis J, Jugurnauth-Little S, Dadaev T, Neal DE, Hamdy FC, et al. Identification of 23 new prostate cancer susceptibility loci using the iCOGS custom genotyping array. NAT GENET. 2013;45:385–391. 391e. - PMC - PubMed
-
- J Schröder FH Hugosson, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis LJ, Recker F, Berenguer A, et al. ERSPC Investigators Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–28. - PubMed
-
- Hodge KK, McNeal JE, Terris MK, Stamey TA. Random systematic versus directed ultrasound guided transrectal core biopsies of the prostate. J Urol. 1989;142:71–74. 74–75. - PubMed
-
- Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T, Zattoni F, Mottet N, European Association of Urology EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65:124–37. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

