Structure and Conformational Properties of d-Glucose/d-Galactose-Binding Protein in Crowded Milieu
- PMID: 28178192
- PMCID: PMC6155729
- DOI: 10.3390/molecules22020244
Structure and Conformational Properties of d-Glucose/d-Galactose-Binding Protein in Crowded Milieu
Abstract
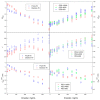
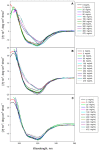
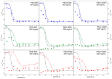
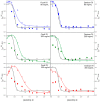
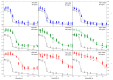
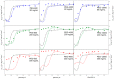
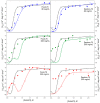
Conformational changes of d-glucose/d-galactose-binding protein (GGBP) were studied under molecular crowding conditions modeled by concentrated solutions of polyethylene glycols (PEG-12000, PEG-4000, and PEG-600), Ficoll-70, and Dextran-70, addition of which induced noticeable structural changes in the GGBP molecule. All PEGs promoted compaction of GGBP and lead to the increase in ordering of its structure. Concentrated solutions of PEG-12000 and PEG-4000 caused GGBP aggregation. Although Ficoll-70 and Dextran-70 also promoted increase in the GGBP ordering, the structural outputs were different for different crowders. For example, in comparison with the GGBP in buffer, the intrinsic fluorescence spectrum of this protein was shifted to short-wave region in the presence of PEGs but was red-shifted in the presence of Ficoll-70 and Dextran-70. It was hypothesized that this difference could be due to the specific interaction of GGBP with the sugar-based polymers (Ficoll-70 and Dextran-70), indicating that protein can adopt different conformations in solutions containing molecular crowders of different chemical nature. It was also shown that all tested crowding agents were able to stabilize GGBP structure shifting the GGBP guanidine hydrochloride (GdnHCl)-induced unfolding curves to higher denaturant concentrations, but their stabilization capabilities did not depend on the hydrodynamic dimensions of the polymers molecules. Refolding of GGBP was complicated by protein aggregation in all tested solutions of crowding agents. The lowest yield of refolded protein was achieved in the highly concentrated solutions of PEG-12000. These data support the previous notion that the influence of macromolecular crowders on proteins is rather complex phenomenon that extends beyond the excluded volume effects.
Keywords: ">d-galactose-binding protein; ">d-glucose/; circular dichroism; intrinsic fluorescence; macromolecular crowding; polymers; protein aggregation; protein folding; protein unfolding.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
The role of water molecules in stereoselectivity of glucose/galactose-binding protein.Sci Rep. 2016 Nov 9;6:36807. doi: 10.1038/srep36807. Sci Rep. 2016. PMID: 27827455 Free PMC article.
-
Conformational stability and domain coupling in D-glucose/D-galactose-binding protein from Escherichia coli.Biochem J. 2004 Jul 1;381(Pt 1):97-103. doi: 10.1042/BJ20040232. Biochem J. 2004. PMID: 15032747 Free PMC article.
-
Carbohydrate affinity for the glucose-galactose binding protein is regulated by allosteric domain motions.J Am Chem Soc. 2012 Dec 5;134(48):19869-76. doi: 10.1021/ja3092938. Epub 2012 Nov 19. J Am Chem Soc. 2012. PMID: 23148479
-
Beyond the excluded volume effects: mechanistic complexity of the crowded milieu.Molecules. 2015 Jan 14;20(1):1377-409. doi: 10.3390/molecules20011377. Molecules. 2015. PMID: 25594347 Free PMC article. Review.
-
Comparative stereochemical analysis of glucose-binding proteins for rational design of glucose-specific agents.J Biomater Sci Polym Ed. 1998;9(4):327-44. doi: 10.1080/09205063.1998.9753059. J Biomater Sci Polym Ed. 1998. PMID: 9586782 Review.
Cited by
-
Cascade Kinetics in an Enzyme-Loaded Aqueous Two-Phase System.Langmuir. 2020 Feb 18;36(6):1401-1408. doi: 10.1021/acs.langmuir.0c00186. Epub 2020 Feb 6. Langmuir. 2020. PMID: 31977224 Free PMC article.
-
Photophysical Properties of BADAN Revealed in the Study of GGBP Structural Transitions.Int J Mol Sci. 2021 Oct 15;22(20):11113. doi: 10.3390/ijms222011113. Int J Mol Sci. 2021. PMID: 34681772 Free PMC article.
-
Glycosylation and Crowded Membrane Effects on Influenza Neuraminidase Stability and Dynamics.J Phys Chem Lett. 2023 Nov 9;14(44):9926-9934. doi: 10.1021/acs.jpclett.3c02524. Epub 2023 Oct 30. J Phys Chem Lett. 2023. PMID: 37903229 Free PMC article.
-
Lipid composition and macromolecular crowding effects on CYP2J2-mediated drug metabolism in nanodiscs.Protein Sci. 2019 May;28(5):928-940. doi: 10.1002/pro.3603. Epub 2019 Apr 1. Protein Sci. 2019. PMID: 30861250 Free PMC article.
-
Single-Molecule Studies on a FRET Biosensor: Lessons from a Comparison of Fluorescent Protein Equipped versus Dye-Labeled Species.Molecules. 2018 Nov 27;23(12):3105. doi: 10.3390/molecules23123105. Molecules. 2018. PMID: 30486450 Free PMC article.
References
-
- Eronina T.B., Chebotareva N.A., Roman S.G., Kleymenov S.Y., Makeeva V.F., Poliansky N.B., Muranov K.O., Kurganov B.I. Thermal denaturation and aggregation of apoform of glycogen phosphorylase b. Effect of crowding agents and chaperones. Biopolymers. 2014;101:504–516. doi: 10.1002/bip.22410. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

