Anti-Inflammatory Activity of Sanghuangporus sanghuang Mycelium
- PMID: 28178212
- PMCID: PMC5343882
- DOI: 10.3390/ijms18020347
Anti-Inflammatory Activity of Sanghuangporus sanghuang Mycelium
Abstract
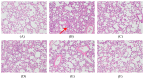
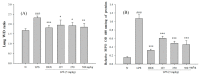
Acute lung injury (ALI) is characterized by inflammation of the lung tissue and oxidative injury caused by excessive accumulation of reactive oxygen species. Studies have suggested that anti-inflammatory or antioxidant agents could be used for the treatment of ALI with a good outcome. Therefore, our study aimed to test whether the mycelium extract of Sanghuangporus sanghuang (SS-1), believed to exhibit antioxidant and anti-inflammatory properties, could be used against the excessive inflammatory response associated with lipopolysaccharides (LPS)-induced ALI in mice and to investigate its possible mechanism of action. The experimental results showed that the administration of SS-1 could inhibit LPS-induced inflammation. SS-1 could reduce the number of inflammatory cells, inhibit myeloperoxidase (MPO) activity, regulate the TLR4/PI3K/Akt/mTOR pathway and the signal transduction of NF-κB and MAPK pathways in the lung tissue, and inhibit high mobility group box-1 protein 1 (HNGB1) activity in BALF. In addition, SS-1 could affect the synthesis of antioxidant enzymes Heme oxygenase 1 (HO-1) and Thioredoxin-1 (Trx-1) in the lung tissue and regulate signal transduction in the KRAB-associated protein-1 (KAP1)/nuclear factor erythroid-2-related factor Nrf2/Kelch Like ECH associated Protein 1 (Keap1) pathway. Histological results showed that administration of SS-1 prior to induction could inhibit the large-scale LPS-induced neutrophil infiltration of the lung tissue. Therefore, based on all experimental results, we propose that SS-1 exhibits a protective effect against LPS-induced ALI in mice. The mycelium of S. sanghuang can potentially be used for the treatment or prevention of inflammation-related diseases.
Keywords: HNGB; HO-1; KAP1/Nrf2 pathway; PI3K/Akt/mTOR pathways; acute lung injury; lipopolysaccharide; mycelium of Sanghuangporus sanghuang.
Conflict of interest statement
The authors declare no conflict of interest.
Figures














References
-
- Huang C.H., Yang M.L., Tsai C.H., Li Y.C., Lin Y.J., Kuan Y.H. Ginkgo biloba leaves extract (EGb-761) attenuates lipopolysaccharide-induced acute lung injury via inhibition of oxidative stress and NF-κB-dependent matrix metalloproteinase-9 pathway. Phytomedicine. 2013;20:303–309. doi: 10.1016/j.phymed.2012.11.004. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

