Extrachromosomal oncogene amplification drives tumour evolution and genetic heterogeneity
- PMID: 28178237
- PMCID: PMC5334176
- DOI: 10.1038/nature21356
Extrachromosomal oncogene amplification drives tumour evolution and genetic heterogeneity
Abstract
Human cells have twenty-three pairs of chromosomes. In cancer, however, genes can be amplified in chromosomes or in circular extrachromosomal DNA (ecDNA), although the frequency and functional importance of ecDNA are not understood. We performed whole-genome sequencing, structural modelling and cytogenetic analyses of 17 different cancer types, including analysis of the structure and function of chromosomes during metaphase of 2,572 dividing cells, and developed a software package called ECdetect to conduct unbiased, integrated ecDNA detection and analysis. Here we show that ecDNA was found in nearly half of human cancers; its frequency varied by tumour type, but it was almost never found in normal cells. Driver oncogenes were amplified most commonly in ecDNA, thereby increasing transcript level. Mathematical modelling predicted that ecDNA amplification would increase oncogene copy number and intratumoural heterogeneity more effectively than chromosomal amplification. We validated these predictions by quantitative analyses of cancer samples. The results presented here suggest that ecDNA contributes to accelerated evolution in cancer.
Figures

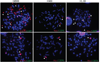





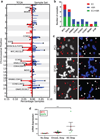
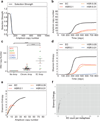
Comment in
-
Cancer genomics: ECdetect hunts extrachromosomal DNA.Nat Rev Genet. 2017 Apr;18(4):212. doi: 10.1038/nrg.2017.13. Epub 2017 Feb 27. Nat Rev Genet. 2017. PMID: 28239156 No abstract available.
References
-
- Stark GR, Debatisse M, Giulotto E, Wahl GM. Recent progress in understanding mechanisms of mammalian DNA amplification. Cell. 1989;57:901–908. - PubMed
-
- Schimke RT. Gene amplification in cultured animal cells. Cell. 1984;37:705–713. - PubMed
-
- Fan Y, et al. Frequency of double minute chromosomes and combined cytogenetic abnormalities and their characteristics. J Appl Genet. 2011;52:53–59. - PubMed
-
- Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23–28. - PubMed
Online Methods References
-
- Lee PM. Bayesian statistics: an introduction. 4th. John Wiley & Sons; 2012.
-
- Bradley D, Roth G. Adaptive thresholding using the integral image. Journal of graphics, gpu, and game tools. 2007;12:13–21.
-
- Lander ES, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

