Surveillance of HIV-1 pol transmitted drug resistance in acutely and recently infected antiretroviral drug-naïve persons in rural western Kenya
- PMID: 28178281
- PMCID: PMC5298248
- DOI: 10.1371/journal.pone.0171124
Surveillance of HIV-1 pol transmitted drug resistance in acutely and recently infected antiretroviral drug-naïve persons in rural western Kenya
Abstract
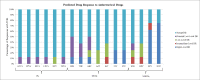
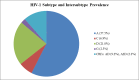
HIV-1 transmitted drug resistance (TDR) is of increasing public health concern in sub-Saharan Africa with the rollout of antiretroviral (ARV) therapy. Such data are, however, limited in Kenya, where HIV-1 drug resistance testing is not routinely performed. From a population-based household survey conducted between September and November 2012 in rural western Kenya, we retrospectively assessed HIV-1 TDR baseline rates, its determinants, and genetic diversity among drug-naïve persons aged 15-59 years with acute HIV-1 infections (AHI) and recent HIV-1 infections (RHI) as determined by nucleic acid amplification test and both Limiting Antigen and BioRad avidity immunoassays, respectively. HIV-1 pol sequences were scored for drug resistance mutations using Stanford HIVdb and WHO 2009 mutation guidelines. HIV-1 subtyping was computed in MEGA6. Eighty seven (93.5%) of the eligible samples were successfully sequenced. Of these, 8 had at least one TDR mutation, resulting in a TDR prevalence of 9.2% (95% CI 4.7-17.1). No TDR was observed among persons with AHI (n = 7). TDR prevalence was 4.6% (95% CI 1.8-11.2) for nucleoside reverse transcriptase inhibitors (NRTIs), 6.9% (95% CI 3.2-14.2) for non- nucleoside reverse transcriptase inhibitors (NNRTIs), and 1.2% (95% CI 0.2-6.2) for protease inhibitors. Three (3.4% 95% CI 0.8-10.1) persons had dual-class NRTI/NNRTI resistance. Predominant TDR mutations in the reverse transcriptase included K103N/S (4.6%) and M184V (2.3%); only M46I/L (1.1%) occurred in the protease. All the eight persons were predicted to have different grades of resistance to the ARV regimens, ranging from potential low-level to high-level resistance. HIV-1 subtype distribution was heterogeneous: A (57.5%), C (6.9%), D (21.8%), G (2.3%), and circulating recombinant forms (11.5%). Only low CD4 count was associated with TDR (p = 0.0145). Our findings warrant the need for enhanced HIV-1 TDR monitoring in order to inform on population-based therapeutic guidelines and public health interventions.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- UNAIDS. Global Report: UNAIDS Report on the Global AIDS Epidemic 2013. 2013.
-
- NASCOP. Kenya AIDS Indicator Survey 2012: Preliminary Report. 2013.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

