Identification of a vacuolar proton channel that triggers the bioluminescent flash in dinoflagellates
- PMID: 28178296
- PMCID: PMC5298346
- DOI: 10.1371/journal.pone.0171594
Identification of a vacuolar proton channel that triggers the bioluminescent flash in dinoflagellates
Abstract
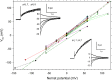
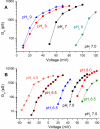
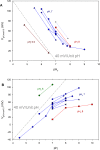
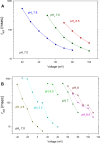
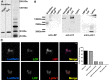
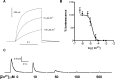
In 1972, J. Woodland Hastings and colleagues predicted the existence of a proton selective channel (HV1) that opens in response to depolarizing voltage across the vacuole membrane of bioluminescent dinoflagellates and conducts protons into specialized luminescence compartments (scintillons), thereby causing a pH drop that triggers light emission. HV1 channels were subsequently identified and demonstrated to have important functions in a multitude of eukaryotic cells. Here we report a predicted protein from Lingulodinium polyedrum that displays hallmark properties of bona fide HV1, including time-dependent opening with depolarization, perfect proton selectivity, and characteristic ΔpH dependent gating. Western blotting and fluorescence confocal microscopy of isolated L. polyedrum scintillons immunostained with antibody to LpHV1 confirm LpHV1's predicted organellar location. Proteomics analysis demonstrates that isolated scintillon preparations contain peptides that map to LpHV1. Finally, Zn2+ inhibits both LpHV1 proton current and the acid-induced flash in isolated scintillons. These results implicate LpHV1 as the voltage gated proton channel that triggers bioluminescence in L. polyedrum, confirming Hastings' hypothesis. The same channel likely mediates the action potential that communicates the signal along the tonoplast to the scintillon.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Fogel M, Hastings JW. Bioluminescence: mechanism and mode of control of scintillon activity. Proc Natl Acad Sci U S A. 1972;69: 690–3. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=426536&tool=pm... - PMC - PubMed
-
- Thomas RC, Meech RW. Hydrogen ion currents and intracellular pH in depolarized voltage-clamped snail neurones. Nature. 1982;299: 826–828. - PubMed
-
- Barish ME, Baud C. A voltage-gated hydrogen ion current in the oocyte membrane of the axolotl, Ambystoma. J Physiol. 1984;352: 243–263. Available: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dop... - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

