Tumor-Penetrating Nanosystem Strongly Suppresses Breast Tumor Growth
- PMID: 28178415
- PMCID: PMC5819594
- DOI: 10.1021/acs.nanolett.6b03815
Tumor-Penetrating Nanosystem Strongly Suppresses Breast Tumor Growth
Abstract
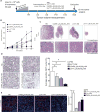
Antiangiogenic and vascular disrupting compounds have shown promise in cancer therapy, but tend to be only partially effective. We previously reported a potent theranostic nanosystem that was highly effective in glioblastoma and breast cancer mouse models, retarding tumor growth and producing some cures [ Agemy , L. et al. Proc. Natl. Acad. Sci. U.S.A. 2011 , 108 , 17450 - 17455 . Agemy , L. et al. Mol. Ther. 2013 , 21 , 2195 - 2204 .]. The nanosystem consists of iron oxide NPs ("nanoworms") coated with a composite peptide with tumor-homing and pro-apoptotic domains. The homing component targets tumor vessels by binding to p32/gC1qR at the surface or tumor endothelial cells. We sought to further improve the efficacy nanosystem by searching for an optimally effective homing peptide that would also incorporate a tumor-penetrating function. To this effect, we tested a panel of candidate p32 binding peptides with a sequence motif that conveys tumor-penetrating activity (CendR motif). We identified a peptide designated as Linear TT1 (Lin TT1) (sequence: AKRGARSTA) as most effective in causing tumor homing and penetration of the nanosystem. This peptide had the lowest affinity for p32 among the peptides tested. The low affinity may have moderated the avidity effect from the multivalent presentation on nanoparticles (NPs), such that the NPs avoid getting trapped by the so-called "binding-site barrier", which can hinder tissue penetration of compounds with a high affinity for their receptors. Treatment of breast cancer mice with the LinTT1 nanosystem showed greatly improved efficacy compared to the original system. These results identify a promising treatment modality and underscore the value of tumor penetration effect in improving the efficacy tumor treatment.
Keywords: C-end Rule; Cancer nanomedicine; iron-oxide nanoparticles; targeted drug delivery; tumor penetrating peptide; vascular disrupting agent.
Conflict of interest statement
Figures





References
-
- Ferrara N, Alitalo K. Nat Med. 1996;5:1359–1364. - PubMed
-
- Hanahan D, Folkman J. Cell. 1996;86:353–364. - PubMed
-
- Pham E, Yin M, Peters CG, Lee CR, Brown D, Xu P, Man S, Jayaraman L, Rohde E, Chow A, Lazarus D, Eliasof S, Foster FS, Kerbel RS. Cancer Res. 2016;76:4493–503. - PubMed
-
- Ellerby HM, Arap W, Ellerby LM, Jain R, Andrusiak R, Rio GD, Krajewski S, Lombardo CR, Rao R, Ruoslahti E, Bredesen DE, Pasqualini R. Nat Med. 1999;9:1032–1038. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

