Thermal Decomposition Synthesis of Iron Oxide Nanoparticles with Diminished Magnetic Dead Layer by Controlled Addition of Oxygen
- PMID: 28178419
- PMCID: PMC6004320
- DOI: 10.1021/acsnano.7b00609
Thermal Decomposition Synthesis of Iron Oxide Nanoparticles with Diminished Magnetic Dead Layer by Controlled Addition of Oxygen
Abstract
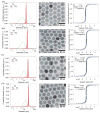
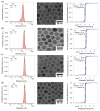
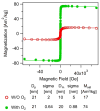
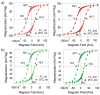
Decades of research focused on size and shape control of iron oxide nanoparticles have led to methods of synthesis that afford excellent control over physical size and shape but comparatively poor control over magnetic properties. Popular synthesis methods based on thermal decomposition of organometallic precursors in the absence of oxygen have yielded particles with mixed iron oxide phases, crystal defects, and poorer than expected magnetic properties, including the existence of a thick "magnetically dead layer" experimentally evidenced by a magnetic diameter significantly smaller than the physical diameter. Here, we show how single-crystalline iron oxide nanoparticles with few defects and similar physical and magetic diameter distributions can be obtained by introducing molecular oxygen as one of the reactive species in the thermal decomposition synthesis. This is achieved without the need for any postsynthesis oxidation or thermal annealing. These results address a significant challenge in the synthesis of nanoparticles with predictable magnetic properties and could lead to advances in applications of magnetic nanoparticles.
Keywords: iron oxide; magnetic dead layer; magnetic diameter; magnetic nanoparticles; oxygen; thermal decomposition.
Conflict of interest statement
The authors declare no competing financial interest.
Figures









References
-
- Cheng G, Walker ARH. Synthesis and Characterization of Cobalt/gold Bimetallic Nanoparticles. J Magn Magn Mater. 2007;311:31–35.
-
- Cheon JW, Kang NJ, Lee SM, Lee JH, Yoon JH, Oh SJ. Shape Evolution of Single-Crystalline Iron Oxide Nanocrystals. J Am Chem Soc. 2003;125:6553–6557. - PubMed
- 2004;126:1950–1951.
-
- Eustis S, El-Sayed MA. Why Gold Nanoparticles are More Precious Than Pretty Gold: Noble Metal Surface Plasmon Resonance and its Enhancement of the Radiative and Nonradiative Properties of Nanocrystals of Different Shapes. Chem Soc Rev. 2006;35:209–217. - PubMed
-
- Gerion D, Pinaud F, Williams SC, Parak WJ, Zanchet D, Weiss S, Alivisatos AP. Synthesis and Properties of Biocompatible Water-soluble Silica-coated CdSe/ZnS Semiconductor Quantum Dots. J Phys Chem B. 2001;105:8861–8871.
-
- Joo J, Yu T, Kim YW, Park HM, Wu FX, Zhang JZ, Hyeon T. Multigrarn Scale Synthesis and Characterization of Monodisperse Tetragonal Zirconia Nanocrystals. J Am Chem Soc. 2003;125:6553–6557. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

