Stromal fibroblasts present in breast carcinomas promote tumor growth and angiogenesis through adrenomedullin secretion
- PMID: 28178651
- PMCID: PMC5362520
- DOI: 10.18632/oncotarget.14999
Stromal fibroblasts present in breast carcinomas promote tumor growth and angiogenesis through adrenomedullin secretion
Abstract
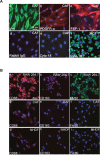
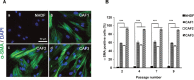
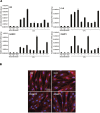
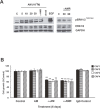
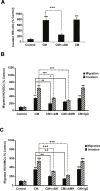
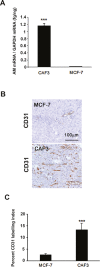
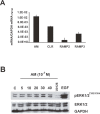
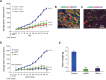
Tumor- or cancer-associated fibroblasts (TAFs or CAFs) are active players in tumorigenesis and exhibit distinct angiogenic and tumorigenic properties. Adrenomedullin (AM), a multifunctional peptide plays an important role in angiogenesis and tumor growth through its receptors calcitonin receptor-like receptor/receptor activity modifying protein-2 and -3 (CLR/RAMP2 and CLR/RAMP3). We show that AM and AM receptors mRNAs are highly expressed in CAFs prepared from invasive breast carcinoma when compared to normal fibroblasts. Immunostaining demonstrates the presence of immunoreactive AM and AM receptors in the CAFs (n = 9). The proliferation of CAFs is decreased by anti-AM antibody (αAM) and anti-AM receptors antibody (αAMR) treatment, suggesting that AM may function as a potent autocrine/paracrine growth factor. Systemic administration of αAMR reduced neovascularization of in vivo Matrigel plugs containing CAFs as demonstrated by reduced numbers of the vessel structures, suggesting that AM is one of the CAFs-derived factors responsible for endothelial cell-like and pericytes recruitment to built a neovascularization. We show that MCF-7 admixed with CAFs generated tumors of greater volume significantly different from the MCF-7 xenografts in nude mice due in part to the induced angiogenesis. αAMR and AM22-52 therapies significantly suppressed the growth of CAFs/MCF-7 tumors. Histological examination of tumors treated with AM22-52 and aAMR showed evidence of disruption of tumor vasculature with depletion of vascular endothelial cells, induced apoptosis and decrease of tumor cell proliferation. Our findings highlight the importance of CAFs-derived AM pathway in growth of breast carcinoma and in neovascularization by supplying and amplifying signals that are essential for pathologic angiogenesis.
Keywords: adrenomedullin; breast cancer; invasion; myofibroblasts; tumor growth.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures

References
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. - PubMed
-
- Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. - PubMed
-
- Jacobs TW, Byrne C, Colditz G, Connolly JL, Schnitt SJ. Radial scars in benign breast-biopsy specimens and the risk of breast cancer. N Engl J Med. 1999;340:430–436. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

