Differential sensitivities of bladder cancer cell lines to resveratol are unrelated to its metabolic profile
- PMID: 28178690
- PMCID: PMC5522211
- DOI: 10.18632/oncotarget.15041
Differential sensitivities of bladder cancer cell lines to resveratol are unrelated to its metabolic profile
Abstract
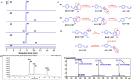
Resveratrol (RV) is a natural polyphenol compound with a wide range of activities, including inhibition of human bladder cancer (HBC) cell growth. Because RV is rapidly metabolized and has poor bioavailability, it is unclear whether the antitumor activity is due to RV or its metabolites. We therefore used liquid chromatography-mass spectroscopy, qRT-PCR, immunocytochemistry and western blotting to evaluate the metabolic profile and biotransformation of RV in the T24 and EJ HBC cell lines. Both T24 and EJ cells generated the same RV metabolite, RV monosulfate (RVS), and both exhibited upregulation of the RV-associated metabolic enzyme SULT1A1 (sulfotransferase). Despite these similarities, T24 cells were more sensitive to RV than EJ cells, yet T24 cells exhibited no sensitivity to an RVS mixture (84.13% RVS). Primary rat bladder epithelial cells showed no adverse effects when exposed to a therapeutic dose (100 μM) of RV. The differences in RV sensitivity between the two HBC cell lines did not reflect differences in the RV metabolic profile or SULT1A1 expression. Because RV exhibited stronger antitumor activity and better safety than RVS, we conclude that RV has significant therapeutic potential for HBC treatment, provided individual differences are considered during clinical research and application.
Keywords: bladder cancer; chemosensitivity; metabolism; resveratrol; sulfotransferase 1A1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Metabolic profile and structure-activity relationship of resveratrol and its analogs in human bladder cancer cells.Cancer Manag Res. 2019 May 21;11:4631-4642. doi: 10.2147/CMAR.S206748. eCollection 2019. Cancer Manag Res. 2019. PMID: 31191024 Free PMC article.
-
Evaluation of resveratrol sensitivities and metabolic patterns in human and rat glioblastoma cells.Cancer Chemother Pharmacol. 2013 Nov;72(5):965-73. doi: 10.1007/s00280-013-2274-y. Epub 2013 Aug 30. Cancer Chemother Pharmacol. 2013. PMID: 23989725
-
Metabolism of resveratrol in breast cancer cell lines: impact of sulfotransferase 1A1 expression on cell growth inhibition.Cancer Lett. 2008 Mar 18;261(2):172-82. doi: 10.1016/j.canlet.2007.11.008. Epub 2007 Dec 20. Cancer Lett. 2008. PMID: 18082939
-
Distinct sulfonation activities in resveratrol-sensitive and resveratrol-insensitive human glioblastoma cells.FEBS J. 2012 Jul;279(13):2381-92. doi: 10.1111/j.1742-4658.2012.08617.x. Epub 2012 May 21. FEBS J. 2012. PMID: 22540632
-
The Metabolic Characteristics and Bioavailability of Resveratrol Based on Metabolic Enzymes.Nutr Rev. 2025 Apr 1;83(4):749-770. doi: 10.1093/nutrit/nuae161. Nutr Rev. 2025. PMID: 39520710 Review.
Cited by
-
Metabolic profile and structure-activity relationship of resveratrol and its analogs in human bladder cancer cells.Cancer Manag Res. 2019 May 21;11:4631-4642. doi: 10.2147/CMAR.S206748. eCollection 2019. Cancer Manag Res. 2019. PMID: 31191024 Free PMC article.
-
The Green Anti-Cancer Weapon. The Role of Natural Compounds in Bladder Cancer Treatment.Int J Mol Sci. 2021 Jul 21;22(15):7787. doi: 10.3390/ijms22157787. Int J Mol Sci. 2021. PMID: 34360552 Free PMC article. Review.
-
4'-Methoxyresveratrol Improves Hepatic Insulin Resistance Induced by a High-Fat-Diet via Anti-Oxidative-Stress Activity.Diabetes Metab Syndr Obes. 2025 Aug 8;18:2739-2752. doi: 10.2147/DMSO.S535103. eCollection 2025. Diabetes Metab Syndr Obes. 2025. PMID: 40799306 Free PMC article.
-
Resveratrol effects in bladder cancer: A mini review.Genet Mol Biol. 2021 Mar 22;44(1):e20200371. doi: 10.1590/1678-4685-GMB-2020-0371. eCollection 2021. Genet Mol Biol. 2021. PMID: 33749701 Free PMC article.
-
Effects of Resveratrol Supplementation on Methotrexate Chemotherapy-Induced Bone Loss.Nutrients. 2017 Mar 9;9(3):255. doi: 10.3390/nu9030255. Nutrients. 2017. PMID: 28282956 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

