Effect of oxidative stress induced by intracranial iron overload on central pain after spinal cord injury
- PMID: 28178997
- PMCID: PMC5299723
- DOI: 10.1186/s13018-017-0526-y
Effect of oxidative stress induced by intracranial iron overload on central pain after spinal cord injury
Abstract
Background: Central pain (CP) is a common clinical problem in patients with spinal cord injury (SCI). Recent studies found the pathogenesis of CP was related to the remodeling of the brain. We investigate the roles of iron overload and subsequent oxidative stress in the remodeling of the brain after SCI.
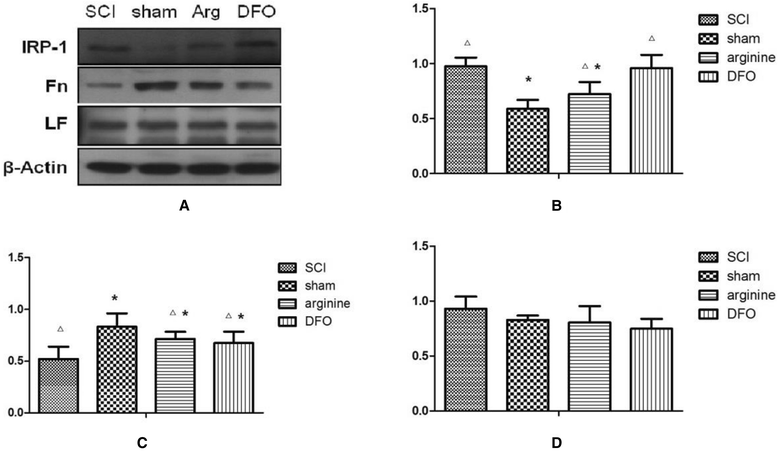
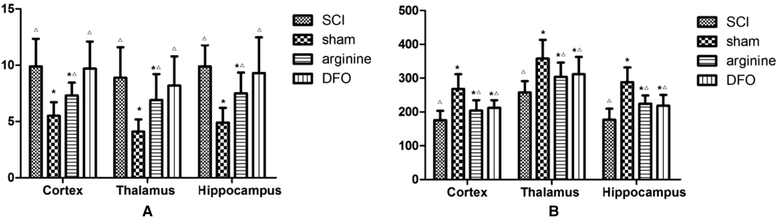
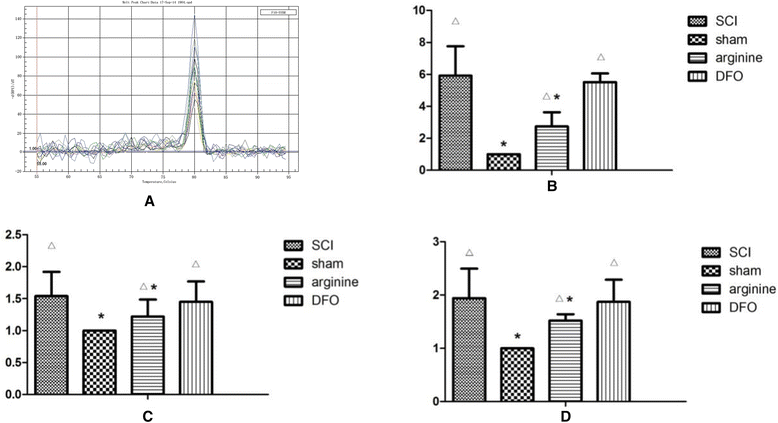
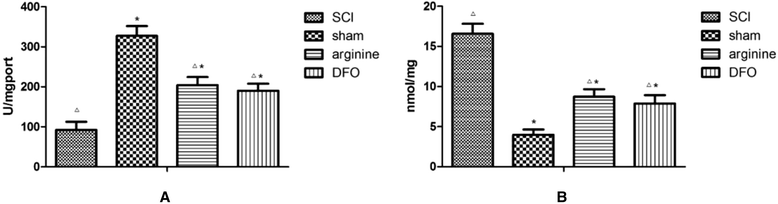
Methods: We established a rat model of central pain after SCI. Rats were divided randomly into four groups: SCI, sham operation, SCI plus deferoxamine (DFX) intervention, and SCI plus nitric oxide synthase (NOS) inhibitor treatment. Pain behavior was observed and thermal pain threshold was measured regularly, and brain levels of iron, transferrin receptor 1 (TfR1), ferritin (Fn), and lactoferrin (Lf), were detected in the different groups 12 weeks after establishment of the model.
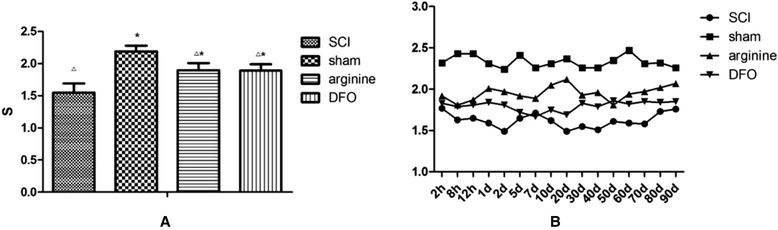
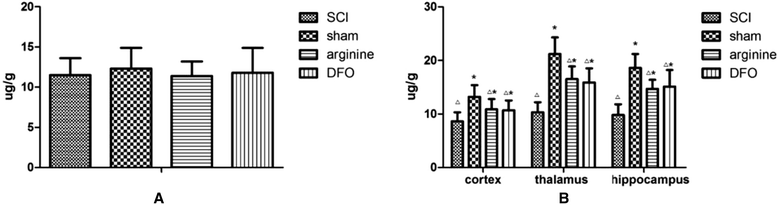
Results: Rats demonstrated self-biting behavior after SCI. Furthermore, the latent period of thermal pain was reduced and iron levels in the hind limb sensory area, hippocampus, and thalamus increased after SCI. Iron-regulatory protein (IRP) 1 levels increased in the hind limb sensory area, while Fn levels decreased. TfR1 mRNA levels were also increased and oxidative stress was activated. Oxidative stress could be inhibited by ferric iron chelators and NOS inhibitors.
Conclusions: SCI may cause intracranial iron overload through the NOS-iron-responsive element/IRP pathway, resulting in central pain mediated by the oxidative stress response. Iron chelators and oxidative stress inhibitors can effectively relieve SCI-associated central pain.
Keywords: Central pain; Iron; Oxidative stress; Spinal cord injury.
Figures
Similar articles
-
In vivo evaluation of microglia activation by intracranial iron overload in central pain after spinal cord injury.J Orthop Surg Res. 2017 May 18;12(1):75. doi: 10.1186/s13018-017-0578-z. J Orthop Surg Res. 2017. PMID: 28521818 Free PMC article.
-
Deferasirox and vitamin D3 co-therapy mitigates iron-induced renal injury by enhanced modulation of cellular anti-inflammatory, anti-oxidative stress, and iron regulatory pathways in rat.J Trace Elem Med Biol. 2022 Dec;74:127085. doi: 10.1016/j.jtemb.2022.127085. Epub 2022 Sep 24. J Trace Elem Med Biol. 2022. PMID: 36179462
-
Psychological stress induces dysregulation of iron metabolism in rat brain.Neuroscience. 2008 Jul 31;155(1):24-30. doi: 10.1016/j.neuroscience.2008.03.091. Epub 2008 Apr 25. Neuroscience. 2008. PMID: 18555617
-
Oxidative stress and antioxidative parameters in patients with spinal cord injury: implications in the pathogenesis of disease.Spinal Cord. 2015 Jan;53(1):3-6. doi: 10.1038/sc.2014.178. Epub 2014 Nov 4. Spinal Cord. 2015. PMID: 25366528 Review.
-
Assessments of sensory plasticity after spinal cord injury across species.Neurosci Lett. 2017 Jun 23;652:74-81. doi: 10.1016/j.neulet.2016.12.031. Epub 2016 Dec 19. Neurosci Lett. 2017. PMID: 28007646 Free PMC article. Review.
Cited by
-
Fibroblast growth factor 21 inhibits ferroptosis following spinal cord injury by regulating heme oxygenase-1.Neural Regen Res. 2024 Jul 1;19(7):1568-1574. doi: 10.4103/1673-5374.387979. Epub 2023 Nov 8. Neural Regen Res. 2024. PMID: 38051901 Free PMC article.
-
Longitudinal differences in iron deposition in periaqueductal gray matter and anterior cingulate cortex are associated with response to erenumab in migraine.Cephalalgia. 2023 Feb;43(2):3331024221144783. doi: 10.1177/03331024221144783. Cephalalgia. 2023. PMID: 36756979 Free PMC article.
-
Dietary intake of diosgenin delays aging of male fish Nothobranchius guentheri through modulation of multiple pathways that play prominent roles in ROS production.Biogerontology. 2022 Apr;23(2):201-213. doi: 10.1007/s10522-022-09955-0. Epub 2022 Jan 31. Biogerontology. 2022. PMID: 35102470
-
Targeting Ferroptosis Attenuates Inflammation, Fibrosis, and Mast Cell Activation in Chronic Prostatitis.J Immunol Res. 2022 Jun 17;2022:6833867. doi: 10.1155/2022/6833867. eCollection 2022. J Immunol Res. 2022. PMID: 35755168 Free PMC article.
-
Main Cations and Cellular Biology of Traumatic Spinal Cord Injury.Cells. 2022 Aug 11;11(16):2503. doi: 10.3390/cells11162503. Cells. 2022. PMID: 36010579 Free PMC article. Review.
References
-
- Bonica JJ. History of pain concepts and pain therapy. Mt Sinai J Med. 1991;58(3):191–202. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

