Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child
- PMID: 28179267
- PMCID: PMC6888512
- DOI: 10.1136/bmj.j1
Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child
Abstract
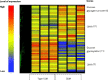
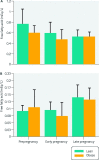
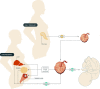
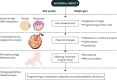
Obesity is the most common medical condition in women of reproductive age. Obesity during pregnancy has short term and long term adverse consequences for both mother and child. Obesity causes problems with infertility, and in early gestation it causes spontaneous pregnancy loss and congenital anomalies. Metabolically, obese women have increased insulin resistance in early pregnancy, which becomes manifest clinically in late gestation as glucose intolerance and fetal overgrowth. At term, the risk of cesarean delivery and wound complications is increased. Postpartum, obese women have an increased risk of venous thromboembolism, depression, and difficulty with breast feeding. Because 50-60% of overweight or obese women gain more than recommended by Institute of Medicine gestational weight guidelines, postpartum weight retention increases future cardiometabolic risks and prepregnancy obesity in subsequent pregnancies. Neonates of obese women have increased body fat at birth, which increases the risk of childhood obesity. Although there is no unifying mechanism responsible for the adverse perinatal outcomes associated with maternal obesity, on the basis of the available data, increased prepregnancy maternal insulin resistance and accompanying hyperinsulinemia, inflammation, and oxidative stress seem to contribute to early placental and fetal dysfunction. We will review the pathophysiology underlying these data and try to shed light on the specific underlying mechanisms.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: The authors have read and understood BMJ policy on declaration of interests and have no conflicts to declare.
Figures


Comment in
-
Clarification of RCOG advice on obese women in pregnancy.BMJ. 2017 Mar 31;356:j1630. doi: 10.1136/bmj.j1630. BMJ. 2017. PMID: 28363895 No abstract available.
-
Author's reply.BMJ. 2017 Mar 31;356:j1631. doi: 10.1136/bmj.j1631. BMJ. 2017. PMID: 28363896 No abstract available.
References
-
- Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 2012;356:491-7. 10.1001/jama.2012.39 pmid:22253363. - DOI - PubMed
-
- Catalano PM. Management of obesity in pregnancy. Obstet Gynecol 2007;356:419-33. 10.1097/01.AOG.0000253311.44696.85 pmid:17267845. - DOI - PubMed
-
- Rasmussen KM, Yaktine AL. Institute of Medicine and National Research Council Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight gain during pregnancy: reexamining the guidelines.National Academies Press, 2009. - PubMed
-
- Aune D, Saugstad OD, Henriksen T, Tonstad S. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. JAMA 2014;356:1536-46. 10.1001/jama.2014.2269 pmid:24737366. - DOI - PubMed
-
- Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005;356:e290-6. 10.1542/peds.2004-1808 pmid:15741354. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
