Evaluation of Plasmodium vivax Cell-Traversal Protein for Ookinetes and Sporozoites as a Preerythrocytic P. vivax Vaccine
- PMID: 28179403
- PMCID: PMC5382829
- DOI: 10.1128/CVI.00501-16
Evaluation of Plasmodium vivax Cell-Traversal Protein for Ookinetes and Sporozoites as a Preerythrocytic P. vivax Vaccine
Abstract
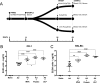
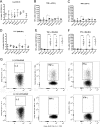
Four different vaccine platforms, each targeting the human malaria parasite Plasmodium vivax cell-traversal protein for ookinetes and sporozoites (PvCelTOS), were generated and assessed for protective efficacy. These platforms consisted of a recombinant chimpanzee adenoviral vector 63 (ChAd63) expressing PvCelTOS (Ad), a recombinant modified vaccinia virus Ankara expressing PvCelTOS (MVA), PvCelTOS conjugated to bacteriophage Qβ virus-like particles (VLPs), and a recombinant PvCelTOS protein expressed in eukaryotic HEK293T cells (protein). Inbred BALB/c mice and outbred CD-1 mice were immunized using the following prime-boost regimens: Ad-MVA, Ad-VLPs, and Ad-protein. Protective efficacy against sporozoite challenge was assessed after immunization using a novel chimeric rodent Plasmodium berghei parasite (Pb-PvCelTOS). This chimeric parasite expresses P. vivax CelTOS in place of the endogenous P. berghei CelTOS and produces fully infectious sporozoites. A single Ad immunization in BALB/c and CD-1 mice induced anti-PvCelTOS antibodies which were boosted efficiently using MVA, VLP, or protein immunization. PvCelTOS-specific gamma interferon- and tumor necrosis factor alpha-producing CD8+ T cells were induced at high frequencies by all prime-boost regimens in BALB/c mice but not in CD-1 mice; in CD-1 mice, they were only marginally increased after boosting with MVA. Despite the induction of anti-PvCelTOS antibodies and PvCelTOS-specific CD8+ T-cell responses, only low levels of protective efficacy against challenge with Pb-PvCelTOS sporozoites were obtained using any immunization strategy. In BALB/c mice, no immunization regimens provided significant protection against a Pb-PvCelTOS chimeric sporozoite challenge. In CD-1 mice, modest protective efficacy against challenge with chimeric P. berghei sporozoites expressing either PvCelTOS or P. falciparum CelTOS was observed using the Ad-protein vaccination regimen.
Keywords: CelTOS; Plasmodium; malaria; preerythrocytic; vaccine; vivax.
Copyright © 2017 Alves et al.
Figures


References
-
- Guerra CA, Howes RE, Patil AP, Gething PW, Van Boeckel TP, Temperley WH, Kabaria CW, Tatem AJ, Manh BH, Elyazar IR, Baird JK, Snow RW, Hay SI. 2010. The international limits and population at risk of Plasmodium vivax transmission in 2009. PLoS Negl Trop Dis 4:e774. doi:10.1371/journal.pntd.0000774. - DOI - PMC - PubMed
-
- RTS,S Clinical Trials Partnership. 2015. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 386:31–45. doi:10.1016/S0140-6736(15)60721-8. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

