SERINC5 protein inhibits HIV-1 fusion pore formation by promoting functional inactivation of envelope glycoproteins
- PMID: 28179429
- PMCID: PMC5392591
- DOI: 10.1074/jbc.M117.777714
SERINC5 protein inhibits HIV-1 fusion pore formation by promoting functional inactivation of envelope glycoproteins
Abstract
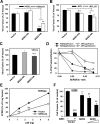
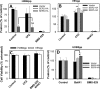
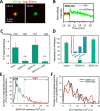
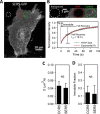
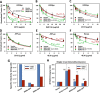
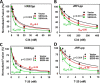
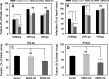
The host proteins, SERINC3 and SERINC5, have been recently shown to incorporate into HIV-1 particles and compromise their ability to fuse with target cells, an effect that is antagonized by the viral Nef protein. Envelope (Env) glycoproteins from different HIV-1 isolates exhibit a broad range of sensitivity to SERINC-mediated restriction, and the mechanism by which SERINCs interfere with HIV-1 fusion remains unclear. Here, we show that incorporation of SERINC5 into virions in the absence of Nef inhibits the formation of small fusion pores between viruses and cells. Strikingly, we found that SERINC5 promotes spontaneous functional inactivation of sensitive but not resistant Env glycoproteins. Although SERINC5-Env interaction was not detected by co-immunoprecipitation, incorporation of this protein enhanced the exposure of the conserved gp41 domains and sensitized the virus to neutralizing antibodies and gp41-derived inhibitory peptides. These results imply that SERINC5 restricts HIV-1 fusion at a step prior to small pore formation by selectively inactivating sensitive Env glycoproteins, likely through altering their conformation. The increased HIV-1 sensitivity to anti-gp41 antibodies and peptides suggests that SER5 also delays refolding of the remaining fusion-competent Env trimers.
Keywords: Env inactivation; HIV neutralization; conformational changes; fluorescence; hemifusion; host defense; membrane fusion; membrane-proximal extracellular domain; peptides; virus entry.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
References
-
- Cavrois M., Neidleman J., Yonemoto W., Fenard D., and Greene W. C. (2004) HIV-1 virion fusion assay: uncoating not required and no effect of Nef on fusion. Virology 328, 36–44 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

