An Evolutionarily Conserved Role for Polydom/Svep1 During Lymphatic Vessel Formation
- PMID: 28179432
- PMCID: PMC5389596
- DOI: 10.1161/CIRCRESAHA.116.308813
An Evolutionarily Conserved Role for Polydom/Svep1 During Lymphatic Vessel Formation
Abstract
Rationale: Lymphatic vessel formation and function constitutes a physiologically and pathophysiologically important process, but its genetic control is not well understood.
Objective: Here, we identify the secreted Polydom/Svep1 protein as essential for the formation of the lymphatic vasculature. We analyzed mutants in mice and zebrafish to gain insight into the role of Polydom/Svep1 in the lymphangiogenic process.
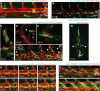
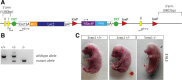
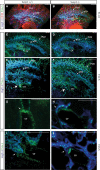
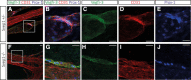
Methods and results: Phenotypic analysis of zebrafish polydom/svep1 mutants showed a decrease in venous and lymphovenous sprouting, which leads to an increased number of intersegmental arteries. A reduced number of primordial lymphatic cells populated the horizontal myoseptum region but failed to migrate dorsally or ventrally, resulting in severe reduction of the lymphatic trunk vasculature. Corresponding mutants in the mouse Polydom/Svep1 gene showed normal egression of Prox-1+ cells from the cardinal vein at E10.5, but at E12.5, the tight association between the cardinal vein and lymphatic endothelial cells at the first lymphovenous contact site was abnormal. Furthermore, mesenteric lymphatic structures at E18.5 failed to undergo remodeling events in mutants and lacked lymphatic valves. In both fish and mouse embryos, the expression of the gene suggests a nonendothelial and noncell autonomous mechanism.
Conclusions: Our data identify zebrafish and mouse Polydom/Svep1 as essential extracellular factors for lymphangiogenesis. Expression of the respective genes by mesenchymal cells in intimate proximity with venous and lymphatic endothelial cells is required for sprouting and migratory events in zebrafish and for remodeling events of the lymphatic intraluminal valves in mouse embryos.
Keywords: Polydom/Svep1; arteries; lymphangiogenesis; lymphatic vessels; mice; veins; zebrafish.
© 2017 The Authors.
Figures
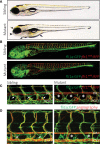
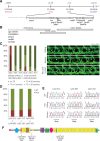
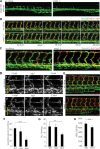
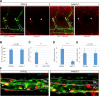
Comment in
-
A New Conserved Player in Lymphatic Morphogenesis.Circ Res. 2017 Apr 14;120(8):1216-1218. doi: 10.1161/CIRCRESAHA.117.310861. Circ Res. 2017. PMID: 28408443 Free PMC article. No abstract available.
Similar articles
-
Svep1 is a binding ligand of Tie1 and affects specific aspects of facial lymphatic development in a Vegfc-independent manner.Elife. 2023 Apr 25;12:e82969. doi: 10.7554/eLife.82969. Elife. 2023. PMID: 37097004 Free PMC article.
-
Polydom Is an Extracellular Matrix Protein Involved in Lymphatic Vessel Remodeling.Circ Res. 2017 Apr 14;120(8):1276-1288. doi: 10.1161/CIRCRESAHA.116.308825. Epub 2017 Feb 8. Circ Res. 2017. PMID: 28179430
-
The adaptor protein Grb2b is an essential modulator for lympho-venous sprout formation in the zebrafish trunk.Angiogenesis. 2021 May;24(2):345-362. doi: 10.1007/s10456-021-09774-w. Epub 2021 Mar 7. Angiogenesis. 2021. PMID: 33677657 Free PMC article.
-
Emerging from the PAC: studying zebrafish lymphatic development.Microvasc Res. 2014 Nov;96:23-30. doi: 10.1016/j.mvr.2014.06.001. Epub 2014 Jun 11. Microvasc Res. 2014. PMID: 24928500 Review.
-
The lymphatic vasculature revisited-new developments in the zebrafish.Methods Cell Biol. 2017;138:221-238. doi: 10.1016/bs.mcb.2016.11.001. Epub 2016 Dec 29. Methods Cell Biol. 2017. PMID: 28129845 Review.
Cited by
-
Recent Advances in Developmental Hematopoiesis: Diving Deeper With New Technologies.Front Immunol. 2021 Nov 24;12:790379. doi: 10.3389/fimmu.2021.790379. eCollection 2021. Front Immunol. 2021. PMID: 34899758 Free PMC article. Review.
-
Cellular crosstalk regulates the aqueous humor outflow pathway and provides new targets for glaucoma therapies.Nat Commun. 2021 Oct 18;12(1):6072. doi: 10.1038/s41467-021-26346-0. Nat Commun. 2021. PMID: 34663817 Free PMC article.
-
SVEP1 as a Genetic Modifier of TEK-Related Primary Congenital Glaucoma.Invest Ophthalmol Vis Sci. 2020 Oct 1;61(12):6. doi: 10.1167/iovs.61.12.6. Invest Ophthalmol Vis Sci. 2020. PMID: 33027505 Free PMC article.
-
Endothelial Cell Development and Its Application to Regenerative Medicine.Circ Res. 2019 Aug 2;125(4):489-501. doi: 10.1161/CIRCRESAHA.119.311405. Epub 2019 Aug 1. Circ Res. 2019. PMID: 31518171 Free PMC article. Review.
-
Svep1 is a binding ligand of Tie1 and affects specific aspects of facial lymphatic development in a Vegfc-independent manner.Elife. 2023 Apr 25;12:e82969. doi: 10.7554/eLife.82969. Elife. 2023. PMID: 37097004 Free PMC article.
References
-
- Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011;7:1371–1380. doi: 10.1038/nm.2545. - PubMed
-
- Ny A, Koch M, Schneider M, et al. A genetic Xenopus laevis tadpole model to study lymphangiogenesis. Nat Med. 2005;11:998–1004. doi: 10.1038/nm1285. - PubMed
-
- Küchler AM, Gjini E, Peterson-Maduro J, Cancilla B, Wolburg H, Schulte-Merker S. Development of the zebrafish lymphatic system requires VEGFC signaling. Curr Biol. 2006;16:1244–1248. doi: 10.1016/j.cub.2006.05.026. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

