Ninein is essential for apico-basal microtubule formation and CLIP-170 facilitates its redeployment to non-centrosomal microtubule organizing centres
- PMID: 28179500
- PMCID: PMC5356440
- DOI: 10.1098/rsob.160274
Ninein is essential for apico-basal microtubule formation and CLIP-170 facilitates its redeployment to non-centrosomal microtubule organizing centres
Abstract
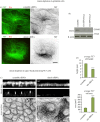
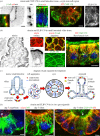
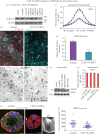
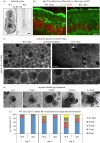
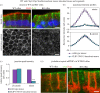
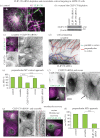
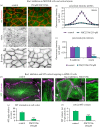
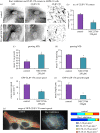
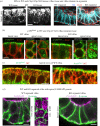
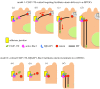
Differentiation of columnar epithelial cells involves a dramatic reorganization of the microtubules (MTs) and centrosomal components into an apico-basal array no longer anchored at the centrosome. Instead, the minus-ends of the MTs become anchored at apical non-centrosomal microtubule organizing centres (n-MTOCs). Formation of n-MTOCs is critical as they determine the spatial organization of MTs, which in turn influences cell shape and function. However, how they are formed is poorly understood. We have previously shown that the centrosomal anchoring protein ninein is released from the centrosome, moves in a microtubule-dependent manner and accumulates at n-MTOCs during epithelial differentiation. Here, we report using depletion and knockout (KO) approaches that ninein expression is essential for apico-basal array formation and epithelial elongation and that CLIP-170 is required for its redeployment to n-MTOCs. Functional inhibition also revealed that IQGAP1 and active Rac1 coordinate with CLIP-170 to facilitate microtubule plus-end cortical targeting and ninein redeployment. Intestinal tissue and in vitro organoids from the Clip1/Clip2 double KO mouse with deletions in the genes encoding CLIP-170 and CLIP-115, respectively, confirmed requirement of CLIP-170 for ninein recruitment to n-MTOCs, with possible compensation by other anchoring factors such as p150Glued and CAMSAP2 ensuring apico-basal microtubule formation despite loss of ninein at n-MTOCs.
Keywords: CLIP-170; IQGAP1; Rac1; microtubules; ninein; non-centrosomal MTOCs.
© 2017 The Authors.
Figures


References
-
- Bacallao R, Antony C, Dotti C, Karsenti E, Stelzer EH, Simons K. 1989. The subcellular organization of Madin-Darby canine kidney cells during the formation of a polarized epithelium. J. Cell Biol. 109, 2817–2832. (doi:10.1083/jcb.109.6.2817) - DOI - PMC - PubMed
-
- Meads T, Schroer TA. 1995. Polarity and nucleation of microtubules in polarized epithelial cells. Cell Motil. Cytoskeleton 32, 273–288. (doi:10.1002/cm.970320404) - DOI - PubMed
-
- Mogensen MM, Tucker JB, Stebbings H. 1989. Microtubule polarities indicate that nucleation and capture of microtubules occurs at cell surfaces in Drosophila. J. Cell Biol. 108, 1445–1452. (doi:10.1083/jcb.108.4.1445) - DOI - PMC - PubMed
-
- Sanchez AD, Feldman JL. In press. Microtubule-organizing centers: from the centrosome to non-centrosomal sites. Curr. Opin. Cell Biol. (doi:10.1016/j.ceb.2016.09.003) - DOI - PMC - PubMed
-
- Mogensen MM, Tucker JB, Mackie JB, Prescott AR, Nathke IS. 2002. The adenomatous polyposis coli protein unambiguously localizes to microtubule plus ends and is involved in establishing parallel arrays of microtubule bundles in highly polarized epithelial cells. J. Cell Biol. 157, 1041–1048. (doi:10.1083/jcb.200203001) - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

