Oncogenomic disruptions in arsenic-induced carcinogenesis
- PMID: 28179585
- PMCID: PMC5421966
- DOI: 10.18632/oncotarget.15106
Oncogenomic disruptions in arsenic-induced carcinogenesis
Abstract
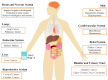
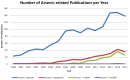
Chronic exposure to arsenic affects more than 200 million people worldwide, and has been associated with many adverse health effects, including cancer in several organs. There is accumulating evidence that arsenic biotransformation, a step in the elimination of arsenic from the human body, can induce changes at a genetic and epigenetic level, leading to carcinogenesis. At the genetic level, arsenic interferes with key cellular processes such as DNA damage-repair and chromosomal structure, leading to genomic instability. At the epigenetic level, arsenic places a high demand on the cellular methyl pool, leading to global hypomethylation and hypermethylation of specific gene promoters. These arsenic-associated DNA alterations result in the deregulation of both oncogenic and tumour-suppressive genes. Furthermore, recent reports have implicated aberrant expression of non-coding RNAs and the consequential disruption of signaling pathways in the context of arsenic-induced carcinogenesis. This article provides an overview of the oncogenomic anomalies associated with arsenic exposure and conveys the importance of non-coding RNAs in the arsenic-induced carcinogenic process.
Keywords: arsenic; cancer; epigenetics; genetics; non-coding RNA.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


References
-
- Sankpal UT, Pius H, Khan M, Shukoor MI, Maliakal P, Lee CM, Abdelrahim M, Connelly SF, Basha R. Environmental factors in causing human cancers: emphasis on tumorigenesis. Tumour biology. 2012;33:1265–1274. - PubMed
-
- Ng JC, Wang J, Shraim A. A global health problem caused by arsenic from natural sources. Chemosphere. 2003;52:1353–1359. - PubMed
-
- Huang L, Wu H, van der Kuijp TJ. The health effects of exposure to arsenic-contaminated drinking water: a review by global geographical distribution. International journal of environmental health research. 2015;25:432–452. - PubMed
-
- Bhattacharjee P, Chatterjee D, Singh KK, Giri AK. Systems biology approaches to evaluate arsenic toxicity and carcinogenicity: an overview. International journal of hygiene and environmental health. 2013;216:574–586. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

