A homolog of cyclophilin D is expressed in Trypanosoma cruzi and is involved in the oxidative stress-damage response
- PMID: 28179991
- PMCID: PMC5292771
- DOI: 10.1038/cddiscovery.2016.92
A homolog of cyclophilin D is expressed in Trypanosoma cruzi and is involved in the oxidative stress-damage response
Abstract
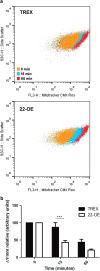
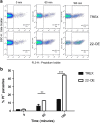
Mitochondria have an important role in energy production, homeostasis and cell death. The opening of the mitochondrial permeability transition pore (mPTP) is considered one of the key events in apoptosis and necrosis, modulated by cyclophilin D (CyPD), a crucial component of this protein complex. In Trypanosoma cruzi, the protozoan parasite that causes Chagas disease, we have previously described that mitochondrial permeability transition occurs after oxidative stress induction in a cyclosporin A-dependent manner, a well-known cyclophilin inhibitor. In the present work, a mitochondrial parasite cyclophilin, named TcCyP22, which is homolog to the mammalian CyPD was identified. TcCyP22-overexpressing parasites showed an enhanced loss of mitochondrial membrane potential and loss of cell viability when exposed to a hydrogen peroxide stimulus compared with control parasites. Our results describe for the first time in a protozoan parasite that a mitochondrial cyclophilin is a component of the permeability transition pore and is involved in regulated cell death induced by oxidative stress.
Figures



Similar articles
-
The cyclophilin D (CypD) of Toxoplasma gondii is involved in the parasite's response to oxidative stress damage.Parasitol Res. 2024 Dec 4;123(12):404. doi: 10.1007/s00436-024-08412-w. Parasitol Res. 2024. PMID: 39627473
-
A Functional Analysis of the Cyclophilin Repertoire in the Protozoan Parasite Trypanosoma Cruzi.Biomolecules. 2018 Oct 31;8(4):132. doi: 10.3390/biom8040132. Biomolecules. 2018. PMID: 30384485 Free PMC article. Review.
-
Oxidative stress damage in the protozoan parasite Trypanosoma cruzi is inhibited by Cyclosporin A.Parasitology. 2015 Jul;142(8):1024-32. doi: 10.1017/S0031182015000232. Epub 2015 Mar 31. Parasitology. 2015. PMID: 25823521
-
Trypanosoma cruzi infection in Cyclophilin D deficient mice.Exp Parasitol. 2021 Jan;220:108044. doi: 10.1016/j.exppara.2020.108044. Epub 2020 Nov 27. Exp Parasitol. 2021. PMID: 33253715
-
Unlocking the Door to Neuronal Woes in Alzheimer's Disease: Aβ and Mitochondrial Permeability Transition Pore.Pharmaceuticals (Basel). 2010 Jun 14;3(6):1936-1948. doi: 10.3390/ph3061936. Pharmaceuticals (Basel). 2010. PMID: 27713335 Free PMC article. Review.
Cited by
-
Theileria annulata Cyclophilin1 (TaCyp1) Interacts With Host Cell MED21.Front Microbiol. 2018 Dec 3;9:2973. doi: 10.3389/fmicb.2018.02973. eCollection 2018. Front Microbiol. 2018. PMID: 30559736 Free PMC article.
-
Mitochondrial permeability transition in protozoan parasites: what we learned from Trypanosoma cruzi.Cell Death Dis. 2017 Sep 21;8(9):e3057. doi: 10.1038/cddis.2017.431. Cell Death Dis. 2017. PMID: 28933785 Free PMC article. No abstract available.
-
Biochemical, Immunohistochemical, Histopathological, and Apoptotic Evaluation of Nickel Oxide Nanoparticle- and Microparticle-Induced Testicular Toxicity in Male Rats.ACS Omega. 2024 Dec 18;9(52):50910-50921. doi: 10.1021/acsomega.4c01005. eCollection 2024 Dec 31. ACS Omega. 2024. PMID: 39758642 Free PMC article.
-
The cyclophilin D (CypD) of Toxoplasma gondii is involved in the parasite's response to oxidative stress damage.Parasitol Res. 2024 Dec 4;123(12):404. doi: 10.1007/s00436-024-08412-w. Parasitol Res. 2024. PMID: 39627473
-
A Functional Analysis of the Cyclophilin Repertoire in the Protozoan Parasite Trypanosoma Cruzi.Biomolecules. 2018 Oct 31;8(4):132. doi: 10.3390/biom8040132. Biomolecules. 2018. PMID: 30384485 Free PMC article. Review.
References
-
- Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev 2007; 87: 99–163. - PubMed
-
- Brenner C, Grimm S. The permeability transition pore complex in cancer cell death. Oncogene 2006; 25: 4744–4756. - PubMed
-
- Galluzzi L, Kepp O, Krautwal S, Kroemer G, Linkermann A. Molecular mechanisms of regulated necrosis. Semin Cell Dev Biol 2014; 35: 24–32. - PubMed
-
- Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol 2010; 11: 621–632. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

