JNK at the crossroad of obesity, insulin resistance, and cell stress response
- PMID: 28180059
- PMCID: PMC5279903
- DOI: 10.1016/j.molmet.2016.12.001
JNK at the crossroad of obesity, insulin resistance, and cell stress response
Abstract
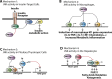
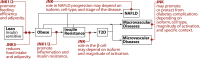
Background: The cJun-N-terminal-kinase (JNK) plays a central role in the cell stress response, with outcomes ranging from cell death to cell proliferation and survival, depending on the specific context. JNK is also one of the most investigated signal transducers in obesity and insulin resistance, and studies have identified new molecular mechanisms linking obesity and insulin resistance. Emerging evidence indicates that whereas JNK1 and JNK2 isoforms promote the development of obesity and insulin resistance, JNK3 activity protects from excessive adiposity. Furthermore, current evidence indicates that JNK activity within specific cell types may, in specific stages of disease progression, promote cell tolerance to the stress associated with obesity and type-2 diabetes.
Scope of review: This review provides an overview of the current literature on the role of JNK in the progression from obesity to insulin resistance, NAFLD, type-2 diabetes, and diabetes complications.
Major conclusion: Whereas current evidence indicates that JNK1/2 inhibition may improve insulin sensitivity in obesity, the role of JNK in the progression from insulin resistance to diabetes, and its complications is largely unresolved. A better understanding of the role of JNK in the stress response to obesity and type-2 diabetes, and the development of isoform-specific inhibitors with specific tissue distribution will be necessary to exploit JNK as possible drug target for the treatment of type-2 diabetes.
Keywords: Autophagy; Diabetes; Endoplasmic eeticulum stress; Inflammation; MAPK; Oxidative stress.
Figures

Similar articles
-
Emerging role of JNK in insulin resistance.Curr Diabetes Rev. 2013 Sep;9(5):422-8. doi: 10.2174/15733998113099990074. Curr Diabetes Rev. 2013. PMID: 23865415 Review.
-
Activated Ask1-MKK4-p38MAPK/JNK stress signaling pathway in human omental fat tissue may link macrophage infiltration to whole-body Insulin sensitivity.J Clin Endocrinol Metab. 2009 Jul;94(7):2507-15. doi: 10.1210/jc.2009-0002. Epub 2009 Apr 7. J Clin Endocrinol Metab. 2009. PMID: 19351724
-
Oxidative stress, ER stress, and the JNK pathway in type 2 diabetes.J Mol Med (Berl). 2005 Jun;83(6):429-39. doi: 10.1007/s00109-005-0640-x. Epub 2005 Mar 10. J Mol Med (Berl). 2005. PMID: 15759102
-
Insulin Resistance, Obesity and Lipotoxicity.Adv Exp Med Biol. 2017;960:277-304. doi: 10.1007/978-3-319-48382-5_12. Adv Exp Med Biol. 2017. PMID: 28585204 Review.
-
Brain JNK and metabolic disease.Diabetologia. 2021 Feb;64(2):265-274. doi: 10.1007/s00125-020-05327-w. Epub 2020 Nov 16. Diabetologia. 2021. PMID: 33200240 Review.
Cited by
-
Nutrition at the Intersection between Gut Microbiota Eubiosis and Effective Management of Type 2 Diabetes.Nutrients. 2024 Jan 16;16(2):269. doi: 10.3390/nu16020269. Nutrients. 2024. PMID: 38257161 Free PMC article. Review.
-
The Role of cGAS-STING Signalling in Metabolic Diseases: from Signalling Networks to Targeted Intervention.Int J Biol Sci. 2024 Jan 1;20(1):152-174. doi: 10.7150/ijbs.84890. eCollection 2024. Int J Biol Sci. 2024. PMID: 38164186 Free PMC article. Review.
-
JNK Regulation of Depression and Anxiety.Brain Plast. 2018 Aug 10;3(2):145-155. doi: 10.3233/BPL-170062. Brain Plast. 2018. PMID: 30151339 Free PMC article. Review.
-
Ectodysplasin A/Ectodysplasin A Receptor System and Their Roles in Multiple Diseases.Front Physiol. 2021 Dec 6;12:788411. doi: 10.3389/fphys.2021.788411. eCollection 2021. Front Physiol. 2021. PMID: 34938205 Free PMC article. Review.
-
MKP1 promotes nonalcoholic steatohepatitis by suppressing AMPK activity through LKB1 nuclear retention.Nat Commun. 2023 Sep 5;14(1):5405. doi: 10.1038/s41467-023-41145-5. Nat Commun. 2023. PMID: 37669951 Free PMC article.
References
-
- Chang L., Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. - PubMed
-
- Hibi M., Lin A., Smeal T., Minden A., Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes & Development. 1993;7:2135–2148. - PubMed
-
- Solinas G., Karin M. JNK1 and IKKbeta: molecular links between obesity and metabolic dysfunction. FASEB Journal. 2010;24:2596–2611. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

