HIV-related proteins prolong macrophage survival through induction of Triggering receptor expressed on myeloid cells-1
- PMID: 28181540
- PMCID: PMC5299418
- DOI: 10.1038/srep42028
HIV-related proteins prolong macrophage survival through induction of Triggering receptor expressed on myeloid cells-1
Abstract
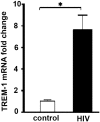
Triggering receptor expressed on myeloid cells-1(TREM-1) is a member of the superimmunoglobulin receptor family. We have previously shown that TREM-1 prolongs survival of macrophages treated with lipoolysaccharide through Egr2-Bcl2 signaling. Recent studies suggest a role for TREM-1 in viral immunity. Human immunodeficiency virus-1 (HIV) targets the monocyte/macrophage lineage at varying stages of infection. Emerging data suggest that macrophages are key reservoirs for latent HIV even in individuals on antiretroviral therapy. Here, we investigated the potential role of TREM-1 in HIV latency in macrophages. Our data show that human macrophages infected with HIV show an increased expression of TREM-1. In parallel, direct exposure to the HIV-related proteins Tat or gp120 induces TREM-1 expression in macrophages and confers anti-apoptotic attributes.NF-κB p65 silencing identified that these proteins induce TREM-1 in p65-dependent manner. TREM-1 silencing in macrophages exposed to HIV-related proteins led to increased caspase 3 activation and reduced Bcl-2 expression, rendering them susceptible to apotosis. These novel data reveal that TREM-1 may play a critical role in establishing HIV reservoir in macrophages by inhibiting apoptosis. Therefore, targeting TREM-1 could be a novel therapeutic approach to enhance clearance of the HIV reservoir, at least within the macrophage pools.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

